Abstract
Purpose
Polypharmacy is associated with negative health outcomes and decreased medication adherence. Polypharmacy is common in cancer populations, but few studies have evaluated the relationship between polypharmacy and aromatase inhibitor (AI) adherence. No studies have evaluated the relationship between over-the-counter (OTC) supplements and AI adherence. Our primary hypothesis was that polypharmacy would be associated with increased risk of premature AI discontinuation.
Methods
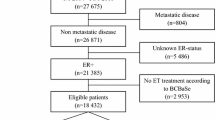
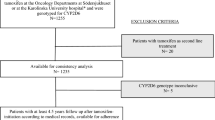
This exploratory analysis used data from the Exemestane and Letrozole Pharmacogenetics (ELPh) trial, a prospective, multicenter, randomized controlled trial that enrolled participants from 2005 to 2009. Included patients were female, postmenopausal, with stage 0–III breast cancer, who had completed indicated chemotherapy, surgery, and radiation. Participants were randomized to adjuvant exemestane or letrozole and completed serial clinical examinations and questionnaires for two years. Concomitant medication data were collected prospectively. Cox proportion models were used for statistical analysis of the relationship between polypharmacy, OTCs, medication class, and AI adherence.
Results
In the 490 analyzed participants, use of any prescription medications at baseline was associated with decreased risk of premature AI discontinuation (HR 0.56, p = 0.02). Use of selective serotonin reuptake inhibitors (SSRIs) or selective serotonin and norepinephrine reuptake inhibitors (SNRIs) at baseline was associated with decreased risk of premature AI discontinuation (HR 0.67, p = 0.04). Use of any OTCs was not associated with AI discontinuation.
Conclusion
Baseline use of prescription medications but not OTCs was associated with increased AI persistence. Future research is needed to understand how this can be utilized to promote AI adherence.
Similar content being viewed by others
Data availability
The data analyzed in this study are available from the corresponding author on reasonable request.
Abbreviations
- AI:
-
Aromatase inhibitor
- AIMSS:
-
Aromatase inhibitor-associated musculoskeletal symptoms
- BCPT:
-
Breast Cancer Prevention Trial
- BMI:
-
Body mass index
- CESD:
-
Center for Epidemiological Studies Depression
- ELPh:
-
Exemestane and Letrozole Pharmacogenetics
- ET:
-
Endocrine therapy
- HADS-A:
-
Hospital Anxiety and Depression Scale-Anxiety
- OTC:
-
Over the counter
- PRO:
-
Patient-reported outcomes
- PSQI:
-
Pittsburgh Sleep Quality Index
- SSRI:
-
Selective serotonin reuptake inhibitor
- SNRI:
-
Selective serotonin and norepinephrine reuptake inhibitor
- VAS:
-
Visual analog scale
References
Murphy CC, Fullington HM, Alvarez CA, Betts AC, Lee SJC, Haggstrom DA, Halm EA (2018) Polypharmacy and patterns of prescription medication use among cancer survivors. Cancer 124(13):2850–2857
Hammersen F, Pursche T, Fischer D, Katalinic A, Waldmann A (2020) Use of complementary and alternative medicine among young patients with breast cancer. Breast Care 15(2):163–170
Betts AC, Murphy CC, Shay LA, Balasubramanian BA, Markham C, Allicock M (2022) Polypharmacy and prescription medication use in a population-based sample of adolescent and young adult cancer survivors. J Cancer Survivorship 17:1149
Shah BM, Hajjar ER (2012) Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med 28(2):173–186
Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM (2016) Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatric Oncol 7(5):346–353
Eyigor S, Kutsal YG, Toraman F, Durmus B, Gokkaya KO, Aydeniz A, Paker N, Borman P (2021) Polypharmacy, physical and nutritional status, and depression in the elderly: do polypharmacy deserve some credits in these problems? Exp Aging Res 47(1):79–91
Nightingale G, Hajjar E, Swartz K, Andrel-Sendecki J, Chapman A (2015) Evaluation of a pharmacist-led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. J Clin Oncol 33(13):1453–1459
Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A et al (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97(1):30–39
Gradishar WJ, Moran MS, Abraham J, Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, et al (2023) NCCN Guidelines Insights: Breast Cancer, Version 4.2023. J Natl Compr Canc Netw 21(6):594–608
Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin S et al (2016) Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 375(3):209–219
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M et al (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28(3):509–518
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 134(2):459–478
Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA et al (2012) Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30(9):936–942
Sweet E, Dowd F, Zhou M, Standish LJ, Andersen MR (2016) The use of complementary and alternative medicine supplements of potential concern during breast cancer chemotherapy. Evid-Based Complement Altern Med 2016:1–8
Zulkipli AF, Islam T, Mohd Taib NA, Dahlui M, Bhoo-Pathy N, Al-Sadat N, Abdul Majid H, Hussain S (2018) Use of complementary and alternative medicine among newly diagnosed breast cancer patients in Malaysia: an early report from the MyBCC study. Integr Cancer Ther 17(2):312–321
Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P et al (2008) Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111(2):365–372
Ganz P, Day R, Ware JJ, Redmond C, Fisher B (1995) Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst 87:18
Radloff LS (1977) The CES-D Scale: a self-report depressionscale for research in the general population. Appl Psychol Meas 1(3):385–401
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213
Kidwell KM, Harte SE, Hayes DF, Storniolo AM, Carpenter J, Flockhart DA, Stearns V, Clauw DJ, Williams DA, Henry NL (2014) Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer 120(16):2403–2411
World Health Organization (2019) Medication safety in polypharmacy. https://www.who.int/publications/i/item/WHO-UHCSDS-2019.11. Accessed 12 October 2023
National Institute on Aging (2021) The dangers of polypharmacy and the case for deprescribing in older adults. https://www.nia.nih.gov/news/dangers-polypharmacy-and-case-deprescribing-olderadults. Accessed 12 October 2023
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99(2):215–220
Yussof I, Mohd Tahir NA, Hatah E, Mohamed Shah N (2022) Factors influencing five-year adherence to adjuvant endocrine therapy in breast cancer patients: a systematic review. The Breast 62:22–35
Calip GS, Xing S, Jun D-H, Lee W-J, Hoskins KF, Ko NY (2017) Polypharmacy and adherence to adjuvant endocrine therapy for breast cancer. J Oncol Pract 13(5):e451–e462
Huiart L, Bouhnik A-D, Rey D, Rousseau F, Retornaz F, Meresse M, Bendiane MK, Viens P, Giorgi R (2013) Complementary or alternative medicine as possible determinant of decreased persistence to aromatase inhibitor therapy among older women with non-metastatic breast cancer. PLoS ONE 8(12):e81677
Trabulsi N, Riedel K, Winslade N, Gregoire JP, Meterissian S, Abrahamovicz M, Tamblyn R, Mayo N, Meguerditchian A (2014) Adherence to anti-estrogen therapy in seniors with breast cancer: how well are we doing? Breast J 20(6):632–638
Wagner LI, Zhao F, Goss PE, Chapman JW, Shepherd LE, Whelan TJ, Mattar BI, Bufill JA, Schultz WC, LaFrancis IE et al (2018) Patient-reported predictors of early treatment discontinuation: treatment-related symptoms and helath-related quality of life among postmenopausal women with primary breast cancer randomized to anastrozole or exemestane on NCIC Clinical Trials Group MA.27 (E1Z03). Breast Cancer Res Treat 169(3):537–548
Lombard JM, Zdenkowski N, Wells K, Beckmore C, Reaby L, Forbes JF, Chirgwin J (2016) Aromatase inhibitor induced musculoskeletal syndrome: a significant problem with limited treatment options. Support Care Cancer 24(5):2139–2146
Gupta A, Henry NL, Loprinzi CL (2020) Management of aromatase inhibitor–induced musculoskeletal symptoms. JCO Oncol Pract 16(11):733–739
Tenti S, Correale P, Cheleschi S, Fioravanti A, Pirtoli L (2020) Aromatase inhibitors-induced musculoskeletal disorders: current knowledge on clinical and molecular aspects. Int J Mol Sci 21(16):5625
Bell SG, Dalton L, Mcneish BL, Fang F, Henry NL, Kidwell KM, Mclean K (2020) Aromatase inhibitor use, side effects and discontinuation rates in gynecologic oncology patients. Gynecol Oncol 159(2):509–514
Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow DM, Sharer CW, Burton GV, Kuzma CS, Moseley A et al (2018) Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor–associated arthralgias in early-stage breast cancer: SWOG S1202. J Clin Oncol 36(4):326–332
Henry NL, Banerjee M, Wicha M, Van Poznak C, Smerage JB, Schott AF, Griggs JJ, Hayes DF (2011) Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer 117(24):5469–5475
Henry NL, Unger JM, Till C, Schott AF, Crew KD, Lew DL, Fisch MJ, Moinpour CM, Wade JL, Hershman DL (2019) Association between body mass index and response to duloxetine for aromatase inhibitor-associated musculoskeletal symptoms in SWOG S1202. Cancer 125(12):2123–2129
Greenlee H, Neugut AI, Falci L, Hillyer GC, Buono D, Mandelblatt JS, Roh JM, Ergas IJ, Kwan ML, Lee M et al (2016) Association between complementary and alternative medicine use and breast cancer chemotherapy initiation. JAMA Oncol 2(9):1170
Boehnke KF, Dean O, Haffajee RL, Hosanagar A (2022) U.S. trends in registration for medical cannabis and reasons for use from 2016 to 2020: an observational study. Ann Intern Med 175(7):945–951
Hasin D, Walsh C (2021) Trends over time in adult cannabis use: a review of recent findings. Curr Opin Psychol 38:80–85
Harrigan M, Mcgowan C, Hood A, Ferrucci LM, Nguyen T, Cartmel B, Li F-Y, Irwin ML, Sanft T (2021) Dietary supplement use and interactions with tamoxifen and aromatase inhibitors in breast cancer survivors enrolled in lifestyle interventions. Nutrients 13(11):3730
Bluethmann SM, Mariotto AB, Rowland JH (2016) Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomark Prev 25(7):1029–1036
Funding
The ELPh trial (ClinicalTrials.gov number: NCT00228956) was supported by a Pharmacogenetics Research Network Grant Number U01 GM61373 (DAF) and Clinical Pharmacology training grants 5T32 GM08425 (DAF) from the National Institute of General Medical Sciences, National Institutes of Health, Bethesda, MD and from grants from Pfizer, Inc. (DFH), Novartis Pharma AG (DFH), and Fashion Footwear Association of New York/QVC Presents Shoes on SaleTM (DFH). The National Center for Research Resources (NCRR) provided grants M01-RR000042 (UM), M01-RR00750 (IU), and M01-RR00052 (JHU). Letrozole and exemestane were provided to study participants by Novartis and Pfizer, respectively. NLH is supported by R01 CA266012 from the National Cancer Institute, National Institutes of Health, Bethesda, MD.
Author information
Authors and Affiliations
Contributions
AMS, DFH, NLH, and VS were responsible for design and conduct of the ELPh clinical trial. EJ and NLH contributed to study conception and design. Data collection, analysis, and preparation were performed by EJ, XT, KMK, and NLH. The first draft of the manuscript was written by EJ and NLH. All authors read and commented on all prior versions of the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
VS has received research grants to her institution from Abbvie, Biocept, Novartis, Pfizer, Puma Biotechnology, and QUE Oncology, she has been a member of the Novartis Advisory Board since October 2021, and she is the Chair of a Data Safety Monitoring Board with AstraZeneca. She additionally has non-financial support with the Foundation Medicine Study Assays. As noted above, DFH reports receiving grants through his institution from the manufacturers of letrozole (Novartis) and exemestane (Pfizer). DFH reports support unrelated to this study but provided to his institution in the last 24 months during conduct and analysis of this study from AstraZeneca, Menarini Silicon Biosystems, Merrimack Pharmaceuticals, and Pfizer. DFH reports personal income related to consulting or advisory board activities from Arvenis, BioVeca, BioTheranostics a Hologic Company, Cellworks, Centrix, Cepheid, EPIC Sciences, EXACT Sciences, Freenome, Guardant, L-Nutra, Macrogenics, Oncocyte, Predictus BioSciences, Stratipath, Tempus, Turnstone Biologics, and Xilis. The University of Michigan holds a patent for which DFH is the named investigator and which was licensed to Menarini Silicon Biosystems, from whom UM and DFH received annual royalties ending 1/1/2022. DFH reports personally held stock options from InBiomotion, Xilis, and from CellWorks. NLH is a consultant for Myovant Pharmaceuticals, a steering committee member for AstraZeneca, and receives royalties from Up-To-Date. The other authors had no disclosures to report.
Ethical approval
The ELPh study received approval from each of the three participating sites: Indiana University Bren and Melvin Simon Cancer Center, the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, and the University of Michigan Comprehensive Cancer Center.
Consent to participate
Informed consent was obtained from all individuals included in the ELPh study for participation and publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joyce, E., Tao, X., Stearns, V. et al. Polypharmacy, over-the-counter medications, and aromatase inhibitor adherence in early-stage breast cancer. Breast Cancer Res Treat 204, 539–546 (2024). https://doi.org/10.1007/s10549-023-07218-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07218-1