Abstract
Purpose
To investigate potential differences in pathological complete response (pCR) rates and overall survival (OS) between HER2-low and HER2-zero patients with early-stage hormone receptor (HR)-positive and triple-negative breast cancer (TNBC), in the neoadjuvant chemotherapy setting.
Methods
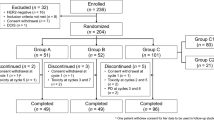
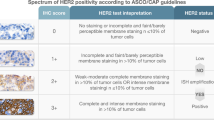
We identified early-stage invasive HER2-negative BC patients who received neoadjuvant chemotherapy diagnosed between 2010 and 2018 in the National Cancer Database. HER2-low was defined by immunohistochemistry (IHC) 1+ or 2+ with negative in situ hybridization, and HER2-zero by IHC0. All the methods were applied separately in the HR-positive and TNBC cohorts. Logistic regression was used to estimate the association of HER2 status with pCR (i.e. ypT0/Tis and ypN0). Kaplan–Meier method and Cox proportional hazards model were applied to estimate the association of HER2 status with OS. Inverse probability weighting and/or multivariable regression were applied to all analyses.
Results
For HR-positive patients, 70.9% (n = 17,934) were HER2-low, whereas 51.1% (n = 10,238) of TNBC patients were HER2-low. For both HR-positive and TNBC cohorts, HER2-low status was significantly associated with lower pCR rates [HR-positive: 5.0% vs. 6.7%; weighted odds ratio (OR) = 0.81 (95% CI: 0.72–0.91), p < 0.001; TNBC: 21.6% vs. 24.4%; weighted OR = 0.91 (95% CI: 0.85–0.98), p = 0.007] and improved OS [HR-positive: weighted hazard ratio = 0.85 (95% CI: 0.79–0.91), p < 0.001; TNBC: weighted hazard ratio = 0.91 (95% CI: 0.86–0.96), p < 0.001]. HER2-low status was associated with favorable OS among patients not achieving pCR [HR-positive: adjusted hazard ratio = 0.83 (95% CI: 0.77–0.89), p < 0.001; TNBC: adjusted hazard ratio = 0.88 (95% CI 0.83–0.94), p < 0.001], while no significant difference in OS was observed in patients who achieved pCR [HR-positive: adjusted hazard ratio = 1.00 (95% CI: 0.61–1.63), p > 0.99; TNBC: adjusted hazard ratio = 1.11 (95% CI: 0.85–1.45), p = 0.44].
Conclusion
In both early-stage HR-positive and TNBC patients, HER2-low status was associated with lower pCR rates. HER2-zero status might be considered an adverse prognostic factor for OS in patients not achieving pCR.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the National Cancer Database, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with the permission of NCDB.
References
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A et al (2022) Breast cancer statistics. CA 72(6):524–541
Knape N, Park JH, Agala CB, Spanheimer P, Morrow M, Downs-Canner S et al (2023) Can we forgo sentinel lymph node biopsy in women aged ≥ 50 years with early-stage hormone-receptor-positive HER2-negative special histologic subtype breast cancer? Ann Surg Oncol 30(2):1042–1050. https://doi.org/10.1245/s10434-022-12626-6
Burguin A, Diorio C, Durocher F (2021) Breast cancer treatments: updates and new challenges. J Pers Med 11(8):808. https://doi.org/10.3390/jpm11080808
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G et al (2017) Adjuvant Pertuzumab and Trastuzumab in early HER2-positive breast cancer. N Engl J Med 377(2):122–131. https://doi.org/10.1056/NEJMoa1703643
Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr et al (2014) Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32(33):3744–3752. https://doi.org/10.1200/jco.2014.55.5730
Fehrenbacher L, Cecchini RS, Geyer CE Jr, Rastogi P, Costantino JP, Atkins JN et al (2020) NSABP B-47/NRG oncology Phase III randomized trial comparing adjuvant chemotherapy with or without Trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and With IHC 1+ or 2. J Clin Oncol 38(5):444–453. https://doi.org/10.1200/jco.19.01455
Gianni L, Lladó A, Bianchi G, Cortes J, Kellokumpu-Lehtinen PL, Cameron DA et al (2010) Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28(7):1131–1137. https://doi.org/10.1200/jco.2009.24.1661
Zhou S, Liu T, Kuang X, Zhen T, Shi H, Lin Y et al (2022) Comparison of clinicopathological characteristics and response to neoadjuvant chemotherapy between HER2-low and HER2-zero breast cancer. Breast 67:1–7. https://doi.org/10.1016/j.breast.2022.12.006
Li Y, Abudureheiyimu N, Mo H, Guan X, Lin S, Wang Z et al (2021) In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: a study of the national cancer center. China Front Oncol 11:774577. https://doi.org/10.3389/fonc.2021.774577
Schettini F, Chic N, Brasó-Maristany F, Paré L, Pascual T, Conte B et al (2021) Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 7(1):1. https://doi.org/10.1038/s41523-020-00208-2
Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K et al (2020) Trastuzumab Deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 382(7):610–621. https://doi.org/10.1056/NEJMoa1914510
Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J et al (2020) Antitumor activity and safety of Trastuzumab Deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol 38(17):1887–1896. https://doi.org/10.1200/jco.19.02318
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E et al (2022) Trastuzumab Deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387(1):9–20. https://doi.org/10.1056/NEJMoa2203690
Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V et al (2019) Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 20(8):1124–1135. https://doi.org/10.1016/s1470-2045(19)30328-6
Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M et al (2021) Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 22(8):1151–1161. https://doi.org/10.1016/s1470-2045(21)00301-6
Tarantino P, Jin Q, Tayob N, Jeselsohn RM, Schnitt SJ, Vincuilla J et al (2022) Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol 8(8):1177–1183. https://doi.org/10.1001/jamaoncol.2022.2286
Kang S, Lee SH, Lee HJ, Jeong H, Jeong JH, Kim JE et al (2022) Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur J Cancer 176:30–40. https://doi.org/10.1016/j.ejca.2022.08.031
Alves FR, Gil L, Vasconcelos de Matos L, Baleiras A, Vasques C, Neves MT et al (2022) Impact of Human Epidermal Growth Factor Receptor 2 (HER2) low status in response to neoadjuvant chemotherapy in early breast cancer. Cureus 14(2):e22330. https://doi.org/10.7759/cureus.22330
de Moura LL, Cesca MG, Tavares MC, Santana DM, Saldanha EF, Guimarães PT et al (2021) HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat 190(1):155–163. https://doi.org/10.1007/s10549-021-06365-7
Shao Y, Yu Y, Luo Z, Guan H, Zhu F, He Y et al (2022) Clinical, pathological complete response, and prognosis characteristics of HER2-low breast cancer in the neoadjuvant chemotherapy setting: a retrospective analysis. Ann Surg Oncol 29(13):8026–8034. https://doi.org/10.1245/s10434-022-12369-4
Wolff AC, Somerfield MR, Dowsett M, Hammond MEH, Hayes DF, McShane LM et al (2023) Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists Guideline Update. J Clin Oncol 41(22):3867–3872. https://doi.org/10.1200/jco.22.02864
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. https://doi.org/10.1038/35021093
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98(19):10869–10874. https://doi.org/10.1073/pnas.191367098
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277. https://doi.org/10.1200/jco.2009.25.9820
Mallin K, Browner A, Palis B, Gay G, McCabe R, Nogueira L et al (2019) Incident cases captured in the national cancer database compared with those in U.S. Population Based Central Cancer Registries in 2012–2014. Ann Surg Oncol 26(6):1604–1612. https://doi.org/10.1245/s10434-019-07213-1
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100(2):229–235. https://doi.org/10.1007/s10549-006-9242-8
Plichta JK, Rushing CN, Lewis HC, Rooney MM, Blazer DG, Thomas SM et al (2023) Implications of missing data on reported breast cancer mortality. Breast Cancer Res Treat 197(1):177–187. https://doi.org/10.1007/s10549-022-06764-4
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG et al (2009) Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338:b2393. https://doi.org/10.1136/bmj.b2393
White IR, Royston P, Wood AM (2011) Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30(4):377–399. https://doi.org/10.1002/sim.4067
Rubin DB (1996) Multiple imputation after 18+ years. J Am Stat Assoc 91(434):473–489
VanderWeele TJ, Ding P (2017) Sensitivity analysis in observational research: introducing the E-Value. Ann Intern Med 167(4):268–274. https://doi.org/10.7326/m16-2607
Haneuse S, VanderWeele TJ, Arterburn D (2019) Using the E-Value to assess the potential effect of unmeasured confounding in observational studies. JAMA 321(6):602–603. https://doi.org/10.1001/jama.2018.21554
Li JJ, Yu Y, Ge J (2023) HER2-low-positive and response to NACT and prognosis in HER2-negative non-metastatic BC. Breast Cancer. https://doi.org/10.1007/s12282-022-01431-4
Almstedt K, Heimes AS, Kappenberg F, Battista MJ, Lehr HA, Krajnak S et al (2022) Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur J Cancer 173:10–19. https://doi.org/10.1016/j.ejca.2022.06.012
Ahn S, Woo JW, Lee K, Park SY (2020) HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. J Pathol Transl Med 54(1):34–44. https://doi.org/10.4132/jptm.2019.11.03
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013. https://doi.org/10.1200/jco.2013.50.9984
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS et al (2018) Human Epidermal Growth Factor Receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 36(20):2105–2122. https://doi.org/10.1200/jco.2018.77.8738
Acknowledgements
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC's NCDB and the hospitals participating in the CoC’s NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
The authors thank Ms. Li Kong, President of Academy of Clinical Research and Study, for coordinating this research work and communication.
Funding
This work was not funded.
Author information
Authors and Affiliations
Contributions
Conception and design: Huiyue Li, Qichen Chen, Jennifer K. Plichta, Sheng Luo, Jian Zhang. Material preparation, data collection and analysis: Huiyue Li, Sheng Luo, Samantha M. Thomas, Jennifer K. Plichta. The first draft of the manuscript was written by Huiyue Li and Yizi Jin, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. The authors have no relevant financial or non-financial interests to disclose. Dr. J. Plichta was the recipient of research funding by the Color Foundation (PI: Plichta). She serves on the National Comprehensive Cancer Network (NCCN) Breast Cancer Screening Committee. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official view of the NIH and other organizations.
Ethical approval
The present study was granted Duke University Institutional Review Board exemption and waiver of informed consent due to the use of de-identified patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Plichta, J.K., Li, K. et al. Impact of HER2-low status for patients with early-stage breast cancer and non-pCR after neoadjuvant chemotherapy: a National Cancer Database Analysis. Breast Cancer Res Treat 204, 89–105 (2024). https://doi.org/10.1007/s10549-023-07171-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07171-z