Abstract
Purpose
Delaying chemotherapy remains a vital goal in therapeutic management of HR+/HER2– metastatic breast cancer (MBC). However, recent reports continue to highlight substantially high chemotherapy utilization in earlier therapy lines. In this study, we explored the impact of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor therapy class, introduced in 2015, on early chemotherapy utilization in an older population of patients with HR+/HER2– MBC in the United States (US).
Methods
Using an interrupted time series design, patients with a confirmed diagnosis of MBC aged ≥ 65 years initiating systemic therapy during 2010–2019 were selected from the SEER-Medicare database. The proportion of chemotherapy use was summarized quarterly based on the date of treatment initiation separately in the first, second, and third lines. Segmented regression models adjusted for autocorrelation over time were fitted to estimate trends before and after the availability of CDK4/6 inhibitors in the first quarter of 2015.
Results
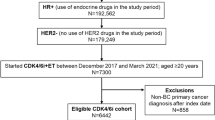
Of the 3244 eligible women (median age at diagnosis: 74 years), all initiated first-line therapy; 47.9% (n = 1581) initiated second-line therapy, and 50.1% (n = 792) initiated third-line therapy. Overall utilization of chemotherapy (alone or in combination) during the study period was 15.7% for the first line, 19.6% for the second line, and 24.8% for the third line. Chemotherapy utilization in the period immediately after introduction of CDK4/6 inhibitor therapy decline by estimated 2.5% in the first line (P = 0.408), 15.5% in the second line (P = 0.005), and 16.3% in the third line (P = 0.003).
Conclusions
This population-based study illustrates that chemotherapy utilization in earlier therapy lines for HR+/HER2– MBC declined steadily between 2010 and 2019. These declines were significantly accelerated by the introduction of CDK4/6 therapy class in 2015, notably in the second- and third-line settings.



Similar content being viewed by others
Data availability
The datasets that support the findings of this study are available from the National Cancer Institute. Restrictions apply to the accessibility of these data, which were purchased and used under license/data use agreement for this study.
References
U.S. Cancer Statistics Working Group. U.S. cancer statistics data visualizations tool, based on 2021 submission data (1999–2019): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. 2022. www.cdc.gov/cancer/dataviz. Accessed 13 July 2022
Gogate A, Wheeler SB, Reeder-Hayes KE, Ekwueme DU, Fairley TL, Drier S et al (2021) Projecting the prevalence and costs of metastatic breast cancer from 2015 through 2030. JNCI Cancer Spectrum 5(4):pkab063
National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: breast cancer. Version 4 [Internet]. 2017 [cited 2022 June 26]. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 13 July 2022.
Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN et al (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J Clin Oncol 34(25):3069–3103
Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E (2012) ESMO guidelines working group locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(7):vii11-9
Barrios HC (2019) The role of chemotherapy in hormone receptor positive advanced breast cancer. GAMO 9(5):215–21
Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ et al (2016) In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: a study of the Southeast Netherlands breast cancer consortium. Ann Oncol 27(2):256–262
Gupta S, Zhang J, Jerusalem G (2014) The association of chemotherapy versus hormonal therapy and health outcomes among patients with hormone receptor-positive, HER2− negative metastatic breast cancer: experience from the patient perspective. Expert Rev Pharmacoecon Outcomes Res 14(6):929–940
Veronesi U, Goldhirsch A, Veronesi P, Gentilini OD, Leonardi MC (eds) (2017) Breast cancer: innovations in research and management. Springer, Switzerland
Brufsky AM (2015) Delaying chemotherapy in the treatment of hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer. Clin Med Insights Oncol 9:CMO-S31586
Goyal RK, Cuyun Carter G, Nagar SP, Nash Smyth E, Price GL, Parikh RC et al (2021) Treatment patterns, adverse events, and direct and indirect economic burden in a privately insured population of patients with HR+/HER2–metastatic breast cancer in the United States. Expert Rev Pharmacoecon Outcomes Res 21(4):699–710
Schleicher SM, Bach PB, Matsoukas K, Korenstein D (2018) Medication overuse in oncology: current trends and future implications for patients and society. Lancet Oncol 19(4):e200–e208
Poorvu PD, Vaz-Luis I, Freedman RA, Lin NU, Barry WT, Winer EP et al (2018) Variation in guideline-concordant care for elderly patients with metastatic breast cancer in the United States. Breast Cancer Res Treat 168(3):727–737
Zanotti G, Hunger M, Perkins JJ, Horblyuk R, Martin M (2017) Treatment patterns and real world clinical outcomes in ER+/HER2-post-menopausal metastatic breast cancer patients in the United States. BMC Cancer 17(1):393
Senkus E, Łacko A (2017) Over-treatment in metastatic breast cancer. Breast 1(31):309–317
Goyal RK, Tzivelekis S, Rothman KJ, Candrilli SD, Kaye JA (2018) Time trends in utilization of G-CSF prophylaxis and risk of febrile neutropenia in a medicare population receiving adjuvant chemotherapy for early-stage breast cancer. Support Care Cancer 26(2):539–548
Gao JJ, Cheng J, Bloomquist E, Sanchez J, Wedam SB, Singh H et al (2020) CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US food and drug administration pooled analysis. Lancet Oncol 21(2):250–260
Carter GC, Sheffield KM, Gossan A, Huang YJ, Zhu YE, Bowman L, et al. (2019) Initial real world treatment patterns and outcomes of abemaciclib for the treatment of HR+, HER2- metastatic breast cancer. Abstract presented at the 2019 San Antonio Breast Cancer Symposium, San Antonio, 11 Dec 2019
Porte B, Carton M, Lerebours F, Brain E, Loirat D, Haroun L et al (2020) Real life efficacy of palbociclib and endocrine therapy in HR positive, HER2 negative advanced breast cancer. Breast 1(54):303–310
Eziokwu AS, Varella L, Kruse ML, Jia X, Moore HC, Budd GT et al (2021) Real-world outcomes of cyclin-dependent kinase inhibitors continued beyond first disease progression in hormone receptor-positive metastatic breast cancer. Clin Breast Cancer 21(3):205–209
Kish JK, Ward MA, Garofalo D, Ahmed HV, McRoy L, Laney J et al (2018) Real-world evidence analysis of palbociclib prescribing patterns for patients with advanced/metastatic breast cancer treated in community oncology practice in the USA one year post approval. Breast Cancer Res 20(1):1–8
Nattinger AB, McAuliffe TL, Schapira MM (1997) Generalizability of the surveillance, epidemiology, and end results registry population: factors relevant to epidemiologic and health care research. J Clin Epidemiol 50(8):939–945
Enewold L, Parsons H, Zhao L, Bott D, Rivera DR, Barrett MJ et al (2020) Updated overview of the SEER-medicare data: enhanced content and applications. JNCI Monographs 2020(55):3–13
Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB et al (2011) Invasive breast cancer. JNCCN 9(2):136–222
National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: breast cancer. Version 1 [Internet]. 2014 [cited 2022 June 26]. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 13 July 2022
Davis KL, Goyal RK, Able SL, Brown J, Li L, Kaye JA (2015) Real-world treatment patterns and costs in a US medicare population with metastatic squamous non-small cell lung cancer. Lung Cancer 87(2):176–185
Goyal RK, Carter GC, Nagar SP, Smyth EN, Price GL, Huang YJ et al (2019) Treatment patterns, survival and economic outcomes in medicare-enrolled, older patients with HR+/HER2− metastatic breast cancer. Curr Med Res Opin 35(10):1699–1710
Vyas A, Gabriel M, Kurian S (2021) Disparities in guideline-concordant initial systemic treatment in women with her2-negative metastatic breast cancer: a SEER-medicare analysis. Breast Cancer Targets Ther 13:259
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The acquisition of SEER-Medicare data for this study was supported by the University of Houston, College of Pharmacy.
Author information
Authors and Affiliations
Contributions
RKG contributed to study design, data analysis, and interpretation, and drafting/revision of the manuscript; HC, SA, HMH, SDC, and MLJ contributed to study design, interpretation of results, and critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was reviewed by the Institutional Review Board at the University of Houston and received “exempt” determination.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goyal, R.K., Holmes, H.M., Chen, H. et al. Impact of CDK4/6 inhibitors on chemotherapy utilization in earlier therapy lines for HR+/HER2– metastatic breast cancer in the United States. Breast Cancer Res Treat 198, 159–166 (2023). https://doi.org/10.1007/s10549-022-06845-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06845-4