Abstract
Objectives
This study aimed to determine whether post-neoadjuvant therapy (NAT) axillary ultrasound (AUS) could reduce the false-negative rate (FNR) of sentinel lymph node biopsy (SLNB). We also performed subgroup analyses to identify the appropriate patient for SLNB.
Methods
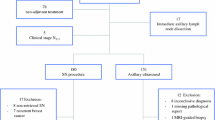
A total of 220 patients with cytologically proven axillary node-positive breast cancer who underwent both SLNB and axillary lymph node dissection (ALND) after NAT were included. We calculated the FNR of SLNB. In the case of post-NAT AUS results available, AUS was classified as negative or positive. Then the FNR of post-NAT AUS combined with SLNB was evaluated. Subgroup analyses based on the number of sentinel lymph nodes removed, molecular subtypes, and the clinical N stage were also performed.
Results
The overall axillary lymph node pathological complete response rate was 45.5% (100/220). The FNR of SLNB alone was 15.8% (95%CI: 9.2 to 22.5%). Post-NAT AUS results were available for 181 patients. When combined negative post-NAT AUS results and SLNB, the FNR was reduced to 7.5% (95%CI: 2.4 to 12.7%). Subgroup analyses of the FNR for SLNB alone and negative post-NAT AUS combined with SLNB were shown as follows: in cases patients with less than three sentinel lymph nodes (SLNs) and at least three SLNs removed, the FNR was decreased from 24.5 to 13.2%, and 9.0 to 5.0%, respectively. The FNR was decreased from 20.8 to 10.5% in HR+/HER2+subgroup, 21.4 to 16.7% in HR−/HER2+subgroup, 15.9 to 7.0% in HR+/HER2− subgroup, and 0% in HR−/HER2− subgroup, respectively. For cN1 patients, the FNR was decreased from 18.1 to 12.1% while 17.1 to 3.6% for cN2 patients and 0% for cN3 patients.
Conclusion
Using negative post-NAT AUS may help to decrease the FNR and improve patient selection for SLNB.


Similar content being viewed by others
Data availability
Due to the privacy of patients, the data related to patients cannot be available for public access but can be obtained from the corresponding author (liangchanghong@gdph.org.cn) on reasonable request approved by the institutional review board of all enrolled centers.
References
Samiei S, Simons JM, Engelen SME, Beets-Tan RGH, Classe JM, Smidt ML, Group E (2021) Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: a systematic review and meta-analysis. JAMA Surg 156(6):e210891
Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Giuliano AE et al (2007) Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American college of surgeons oncology group trial Z0011. J Clin Oncol 25(24):3657–3663
Mamounas EP, Brown A, Anderson S, Smith R, Julian T, Miller B, Bear HD, Caldwell CB, Walker AP, Mikkelson WM et al (2005) Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol 23(12):2694–2702
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, Lebeau A, Liedtke C, von Minckwitz G, Nekljudova V et al (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 14(7):609–618
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Kuerer HM, Bowling M, Flippo-Morton TS et al (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310(14):1455–1461
Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, Meterissian S, Arnaout A, Brackstone M, McCready DR et al (2015) Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 33(3):258–264
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, Bedrosian I, Hobbs BP, DeSnyder SM, Hwang RF et al (2016) Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 34(10):1072–1078
Harvey SC, Wolff AC (2015) Does a picture make a difference? Ultrasound guidance in the management of the axilla after neoadjuvant chemotherapy. J Clin Oncol 33(30):3367–3369
Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE (2017) Sentinel lymph node biopsy for patients with early-stage breast cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol 35(5):561–564
Sun X, Wang XE, Zhang ZP, Shi ZQ, Cong BB, Wang YS, Shao ZM (2020) Neoadjuvant therapy and sentinel lymph node biopsy in HER2-positive breast cancer patients: results from the PEONY trial. Breast Cancer Res Treat 180(2):423–428
Expert Panel on Breast I, Slanetz PJ, Moy L, Baron P, diFlorio RM, Green ED, Heller SL, Holbrook AI, Lee SJ, Lewin AA et al (2017) ACR appropriateness criteria((R)) monitoring response to neoadjuvant systemic therapy for breast cancer. J Am Coll Radiol 14(11S):S462–S475
Kim R, Chang JM, Lee HB, Lee SH, Kim SY, Kim ES, Cho N, Moon WK (2019) Predicting axillary response to neoadjuvant chemotherapy: breast MRI and US in patients with node-positive breast cancer. Radiology 293(1):49–57
Eun NL, Son EJ, Gweon HM, Kim JA, Youk JH (2020) Prediction of axillary response by monitoring with ultrasound and MRI during and after neoadjuvant chemotherapy in breast cancer patients. Eur Radiol 30(3):1460–1469
Chung HL, Le-Petross HT, Leung JWT (2021) Imaging updates to breast cancer lymph node management. Radiographics 41(5):1283–1299
Schwentner L, Helms G, Nekljudova V, Ataseven B, Bauerfeind I, Ditsch N, Fehm T, Fleige B, Hauschild M, Heil J et al (2017) Using ultrasound and palpation for predicting axillary lymph node status following neoadjuvant chemotherapy—results from the multi-center SENTINA trial. Breast 31:202–207
Peppe A, Wilson R, Pope R, Downey K, Rusby J (2017) The use of ultrasound in the clinical re-staging of the axilla after neoadjuvant chemotherapy (NACT). Breast 35:104–108
Samiei S, de Mooij CM, Lobbes MBI, Keymeulen K, van Nijnatten TJA, Smidt ML (2021) Diagnostic performance of noninvasive imaging for assessment of axillary response after neoadjuvant systemic therapy in clinically node-positive breast cancer: a systematic review and meta-analysis. Ann Surg 273(4):694–700
Boughey JC, Ballman KV, Hunt KK, McCall LM, Mittendorf EA, Ahrendt GM, Wilke LG, Le-Petross HT (2015) Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: results from the American college of surgeons oncology group Z1071 trial (alliance). J Clin Oncol 33(30):3386–3393
Morency D, Dumitra S, Parvez E, Martel K, Basik M, Robidoux A, Poirier B, Holloway CMB, Gaboury L, Sideris L et al (2019) Axillary lymph node ultrasound following neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: results from the SN FNAC study. Ann Surg Oncol 26(13):4337–4345
Cao S, Liu X, Cui J, Liu X, Zhong J, Yang Z, Sun D, Wei W (2021) Feasibility and reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients with positive axillary nodes at initial diagnosis: an up-to-date meta-analysis of 3578 patients. Breast 59:256–269
Myers SP, Ahrendt GM, Lee JS, Steiman JG, Soran A, Johnson RR, McAuliffe PF, Diego EJ (2021) Association of tumor molecular subtype and stage with breast and axillary pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol 28(13):8636–8642
Gimbergues P, Abrial C, Durando X, Le Bouedec G, Cachin F, Penault-Llorca F, Mouret-Reynier MA, Kwiatkowski F, Maublant J, Tchirkov A et al (2008) Sentinel lymph node biopsy after neoadjuvant chemotherapy is accurate in breast cancer patients with a clinically negative axillary nodal status at presentation. Ann Surg Oncol 15(5):1316–1321
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD et al (2021) NCCN guidelines(R) insights: breast cancer, version 42021. J Natl Compr Canc Netw 19(5):484–493
Samiei S, van Nijnatten TJA, de Munck L, Keymeulen K, Simons JM, Kooreman LFS, Siesling S, Lobbes MBI, Smidt ML (2020) Correlation between pathologic complete response in the breast and absence of axillary lymph node metastases after neoadjuvant systemic therapy. Ann Surg 271(3):574–580
Nielsen TO, Leung SCY, Rimm DL, Dodson A, Acs B, Badve S, Denkert C, Ellis MJ, Fineberg S, Flowers M et al (2021) Assessment of Ki67 in breast cancer: updated recommendations from the International Ki67 in breast cancer working group. J Natl Cancer Inst 113(7):808–819
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M et al (2010) American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795
Loibl S, Gianni L (2017) HER2-positive breast cancer. Lancet 389(10087):2415–2429
Piltin MA, Hoskin TL, Day CN, Davis J Jr, Boughey JC (2020) Oncologic outcomes of sentinel lymph node surgery after neoadjuvant chemotherapy for node-positive breast cancer. Ann Surg Oncol 27(12):4795–4801
Kahler-Ribeiro-Fontana S, Pagan E, Magnoni F, Vicini E, Morigi C, Corso G, Intra M, Canegallo F, Ratini S, Leonardi MC et al (2021) Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol 47(4):804–812
Barrio AV, Montagna G, Mamtani A, Sevilimedu V, Edelweiss M, Capko D, Cody HS 3rd, El-Tamer M, Gemignani ML, Heerdt A et al (2021) Nodal recurrence in patients with node-positive breast cancer treated with sentinel node biopsy alone after neoadjuvant chemotherapy-a rare event. JAMA Oncol 7(12):1851–1855
Wong SM, Basik M, Florianova L, Margolese R, Dumitra S, Muanza T, Carbonneau A, Ferrario C, Boileau JF (2021) Oncologic safety of sentinel lymph node biopsy alone after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol 28(5):2621–2629
Gerber B, Schneeweiss A, Mobus V, Golatta M, Tesch H, Krug D, Hanusch C, Denkert C, Lubbe K, Heil J et al (2022) Pathological response in the breast and axillary lymph nodes after neoadjuvant systemic treatment in patients with initially node-positive breast cancer correlates with disease free survival: an exploratory analysis of the geparocto trial. Cancers (Basel) 14:3
Kim WH, Kim HJ, Park CS, Lee J, Park HY, Jung JH, Kim WW, Chae YS, Lee SJ, Kim SH (2020) Axillary nodal burden assessed with pretreatment breast MRI Is associated with failed sentinel lymph node identification after neoadjuvant chemotherapy for breast cancer. Radiology 295(2):275–282
Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Feliberti EC, Hunt KK (2016) Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (alliance). Ann Surg 263(4):802–807
Murthy V, Young J, Tokumaru Y, Quinn M, Edge SB, Takabe K (2021) Options to determine pathological response of axillary lymph node metastasis after neoadjuvant chemotherapy in advanced breast cancer. Cancers (Basel) 13:16
Funding
This work was funded by the Key-Area Research and Development Program of Guangdong Province (No.2021B0101420006); National Natural Science Foundation of China (No.82071892, 82271941,82272088, 82171920); Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application (No.2022B1212010011); the National Science Foundation for Young Scientists of China (No.82102019, 82001986); Project Funded by China Postdoctoral Science Foundation (No.2020M682643, 2021M700897); High-level Hospital Construction Project (DFJH201805, DFJHBF202105).
Author information
Authors and Affiliations
Contributions
YL, YW, SF: conceptualization; YL, SF, ZX, XH, MY: methodology; YL, XH, ZX, CL: verification of data; CL, ZL, YW: investigation; MY, CL, XC,PL: visualization; MY, LW, CL: supervision; YL, SF: writing—original draft; CL, ZL, YW, ZX, XH: writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Wang, Y., Feng, S. et al. Axillary ultrasound after neoadjuvant therapy reduces the false-negative rate of sentinel lymph node biopsy in patients with cytologically node-positive breast cancer. Breast Cancer Res Treat 197, 515–523 (2023). https://doi.org/10.1007/s10549-022-06817-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06817-8