Abstract
Purpose
Cardiac function assessment is important for detecting and managing trastuzumab-associated cardiotoxicity. Our study estimates rates and predictors of cardiac assessment among patients receiving trastuzumab for HER2-positive early breast cancer (HER2+EBC) in Australia.
Methods
We conducted a retrospective cohort study of Australians initiating (neo)adjuvant trastuzumab for HER2+EBC between 1 January 2015 and 15 April 2019. We used administrative claims to determine the number of patients receiving guideline-recommended assessment, i.e. evidence of baseline cardiac assessment (between 120 days before and 30 days after trastuzumab initiation) and regular on-treatment cardiac assessments (at least every 120 days). We examined factors associated with baseline and regular on-treatment cardiac assessment.
Results
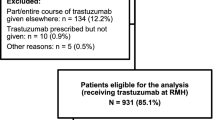
Our study includes 5621 patients (median age 56 years), of whom 4984 (88.7%) had a baseline cardiac function test. Among 4280 patients with at least 12 months of follow-up, 2702 (63.1%) had guideline-recommended cardiac assessment. Rates of guideline-recommended assessment increased with later year of diagnosis (60.9% in 2015 vs 68.3% in 2018, OR 1.34, 95% CI 1.06–1.69). Patients with higher baseline comorbidities and greater socioeconomic disadvantage were less likely to have guideline-recommended cardiac assessment. Cardiac assessment practices varied by State/Territory. There was no association between baseline cardiac risk or anthracycline use and the likelihood of receiving guideline-recommended cardiac assessment.
Conclusion
The majority of patients receiving (neo)adjuvant trastuzumab had guideline-recommended baseline and on-treatment cardiac assessment. Variations in cardiac assessment predominantly related to system-level factors, such as year of diagnosis and geography, rather than individual patient factors.
Similar content being viewed by others
Data availability
We thank the Australian Government Services Australia for providing the data. Access to the datasets analysed during the current study is not permitted without the express permission of the approving human research ethics committees and data custodians.
References
Nemeth BT, Varga ZV, Wu WJ, Pacher P (2017) Trastuzumab cardiotoxicity: from clinical trials to experimental studies. Br J Pharmacol 174(21):3727–3748. https://doi.org/10.1111/bph.13643
Maurea N, Coppola C, Piscopo G, Galletta F, Riccio G, Esposito E, De Lorenzo C, De Laurentiis M, Spallarossa P, Mercuro GJJoCM (2016) Pathophysiology of cardiotoxicity from target therapy and angiogenesis inhibitors. J Cardiovasc Med 17(1):S19–S26
Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, D’Amico R (2012) Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006243.pub2
Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, Smith BD, Hortobagyi GN, Giordano SH (2013) Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol 31(33):4222
Elting LS, Cooksley C, Bekele BN, Frumovitz M, Avritscher EBC, Sun C, Bodurka DC (2006) Generalizability of cancer clinical trial results. Cancer 106(11):2452–2458. https://doi.org/10.1002/cncr.21907
Pearson S-A, Ringland CL, Ward RL (2007) Trastuzumab and metastatic breast cancer: trastuzumab use in Australia—monitoring the effect of an expensive medicine access program. J Clin Oncol 25(24):3688–3693
Lu CY, Srasuebkul P, Drew AK, Ward R, Pearson SA (2012) Positive spillover effects of prescribing requirements: increased cardiac testing in patients treated with trastuzumab for HER2+ metastatic breast cancer. Int Med J 42(11):1229–1235
Lu CY, Srasuebkul P, Drew AK, Chen K, Ward RL, Pearson S-A (2013) Trastuzumab therapy in Australia: which patients with HER2+ metastatic breast cancer are assessed for cardiac function? The Breast 22(4):482–487
Daniels B, Kiely BE, Lord SJ, Houssami N, Lu CY, Ward RL, Pearson S-A (2018) Trastuzumab for metastatic breast cancer: real world outcomes from an Australian whole-of-population cohort (2001–2016). The Breast 38:7–13
Harris CA (2014) Measuring treatment patterns and outcomes of HER2 positive breast cancer using clinical trials and routine clinical care. University of New South Wales, Sydney
Daniels B, Lord SJ, Kiely BE, Houssami N, Haywood P, Lu CY, Ward RL, Pearson S-A (2017) Use and outcomes of targeted therapies in early and metastatic HER2-positive breast cancer in Australia: protocol detailing observations in a whole of population cohort. BMJ open 7(1):e014439
Australian Bureau of Statistics (July, 2016) Australian Geography Standard (ASGS): Volume 5 – remoteness structure. https://www.abs.gov.au/ausstats/abs@.nsf/mf/1270.0.55.005. Accessed 6 Jan 2021
Hugo Centre for Population and Housing (2020) Accessibility/remoteness index of Australia (ARIA). University of Adelaide. https://www.adelaide.edu.au/hugo-centre/services/aria.
Australian Bureau of Statistics (2006) Information paper: an introduction to Socio-Economic Indexes for Areas (SEIFA). Australian Bureau of Statistics. https://www.abs.gov.au/ausstats/abs@.nsf/mf/2039.0 Accessed 6 Jan 2021
Australian Bureau of Statistics (2000) Australian Social Trends. vol ABS cat. no. 4102.0. Australian Bureau of Statistics, Canberra
Schaffer AL, Buckley NA, Dobbins TA, Banks E, Pearson SA (2015) The crux of the matter: did the ABC’s catalyst program change statin use in Australia? Med J Aust 202(11):591–594
Pratt NL, Kerr M, Barratt JD, Kemp-Casey A, Ellett LMK, Ramsay E, Roughead EE (2018) The validity of the rx-risk comorbidity index using medicines mapped to the Anatomical Therapeutic Chemical (ATC) classification system. BMJ open 8(4):e021122
National Breast Cancer Centre (2007) Recommendations for use of trastuzumab for the treatment of HER2-positive breast cancer. Cancer Australia,
Cancer Institute NSW (2006) Cardiac toxicity associated with HER-2 targeted agents. https://www.eviq.org.au/clinical-resources/side-effect-and-toxicity-management/cardiovascular/1852-cardiac-toxicity-associated-with-her-2-target#. Accessed 21 Feb 2020
Srasuebkul P, Dobbins TA, Pearson SA (2014) Validation of a proxy for estrogen receptor status in breast cancer patients using dispensing data. Asia Pac J Clin Oncol 10(2):e63–e68. https://doi.org/10.1111/ajco.12015
Tang M, Schaffer A, Kiely BE, Daniels B, Simes RJ, Lee CK, Pearson S-A (2019) Treatment patterns and survival in HER2-positive early breast cancer: a whole-of-population Australian cohort study (2007–2016). Br J Cancer 121(11):904–911
Chavez-MacGregor M, Niu J, Zhang N, Elting LS, Smith BD, Banchs J, Hortobagyi GN, Giordano SH (2015) Cardiac monitoring during adjuvant trastuzumab-based chemotherapy among older patients with breast cancer. J Clin Oncol 33(19):2176
Henry ML, Niu J, Zhang N, Giordano SH, Chavez-MacGregor M (2018) Cardiotoxicity and cardiac monitoring among chemotherapy-treated breast. Cancer Patients 11(8):1084–1093. https://doi.org/10.1016/j.jcmg.2018.06.005%JJACC:CardiovascularImaging
Dall P, Koch T, Göhler T, Selbach J, Ammon A, Eggert J, Gazawi N, Rezek D, Wischnik A, Hielscher C, Keitel S, Cirrincione U, Hinke A, Feisel-Schwickardi G (2017) Trastuzumab in human epidermal growth factor receptor 2-positive early breast cancer: results of a prospective, noninterventional study on routine treatment between 2006 and 2012 in Germany. Oncologist 22(2):131–138. https://doi.org/10.1634/theoncologist.2016-0193
Kumachev A, Chin-Yee NJ, Yan A, Tomlinson GA, Earle C, Trudeau ME, Ko D, Krzyzanowska MK, Pal R, Brezden CB, Gavura S, Lien K, Chan KK (2013) Impact of physician and center case volume on the adequacy of cardiac monitoring during adjuvant trastuzumab in breast cancer. J Clin Oncol 31(26_suppl):128–128. https://doi.org/10.1200/jco.2013.31.26_suppl.128
Ng D, Ferrusi I, Khong H, Earle C, Trudeau M, Marshall D, Leighl N (2012) Cardiac monitoring during adjuvant trastuzumab therapy for breast cancer. AACR. https://doi.org/10.1158/0008-5472.SABCS12-P5-18-14
Seferina SC, de Boer M, Derksen MW, van den Berkmortel F, van Kampen RJ, van de Wouw AJ, Joore M, Peer PG, Voogd AC, Tjan-Heijnen VC (2016) Cardiotoxicity and cardiac monitoring during adjuvant trastuzumab in daily dutch practice: a study of the southeast netherlands breast cancer consortium. Oncologist 21(5):555–562. https://doi.org/10.1634/theoncologist.2015-0230
Visser A, van de Ven EMW, Ruczynski LIA, Blaisse RJB, van Halteren HK, Aben K, van Laarhoven HWM (2016) Cardiac monitoring during adjuvant trastuzumab therapy: guideline adherence in clinical practice. Acta Oncol 55(4):423–429. https://doi.org/10.3109/0284186X.2015.1068444
Subar M, Lin W, Chen W, Pittman DG (2011) Lack of uniformity in cardiac assessment during trastuzumab therapy. The Breast J 17(4):383–390
Marventano S, Ayala A, Gonzalez N, Rodríguez-Blázquez C, Garcia-Gutierrez S, Forjaz MJ (2014) Multimorbidity and functional status in community-dwelling older adults. Eur J Intern Med 25(7):610–616. https://doi.org/10.1016/j.ejim.2014.06.018
Aarts S, den Akker Mv, Bosma H, Tan F, Verhey F, Metsemakers J, van Boxtel M (2012) The effect of multimorbidity on health related functioning: Temporary or persistent? results from a longitudinal cohort study. J Psychosom Res 73(3):211–217. https://doi.org/10.1016/j.jpsychores.2012.05.014
Veugelers PJ, Yip AM (2003) Socioeconomic disparities in health care use: does universal coverage reduce inequalities in health? J Epidemiol Community Health 57(6):424–428. https://doi.org/10.1136/jech.57.6.424
Demeter S, Reed M, Lix L, MacWilliam L, Leslie WD (2005) Socioeconomic status and the utilization of diagnostic imaging in an urban setting. J Canadian Med Assoc 173(10):1173–1177. https://doi.org/10.1503/cmaj.050609
Quyyumi FF, Wright JD, Accordino MK, Buono D, Law CW, Hillyer GC, Neugut AI, Hershman DL (2019) Factors associated with multidisciplinary consultations in patients with early stage breast cancer. Cancer Invest 37(6):233–241. https://doi.org/10.1080/07357907.2019.1624766
Riba LA, Gruner RA, Alapati A, James TA (2019) Association between socioeconomic factors and outcomes in breast cancer. Breast J 25(3):488–492. https://doi.org/10.1111/tbj.13250
Jong KE, Vale PJ, Armstrong BK (2005) Rural inequalities in cancer care and outcome. Med J Aust 182(1):13
Dasgupta P, Baade PD, Youlden DR, Garvey G, Aitken JF, Wallington I, Chynoweth J, Zorbas H, Youl PH (2018) Variations in outcomes by residential location for women with breast cancer: a systematic review. BMJ open 8(4):e019050–e019050. https://doi.org/10.1136/bmjopen-2017-019050
Services Australia (2020) Medicare statistics. Australian Government. http://medicarestatistics.humanservices.gov.au/. Accessed 4 Sept 2020
Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ (2000) Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur Heart J 21(16):1387–1396
Foley TA, Mankad SV, Anavekar NS, Bonnichsen CR, Morris MF, Miller TD, Araoz PA (2012) Measuring left ventricular ejection fraction-techniques and potential pitfalls. Eur Cardiol Rev 8(2):108–114. https://doi.org/10.15420/ecr.2012.8.2.108
Dang CT, Yu AF, Jones LW, Liu J, Steingart RM, Argolo DF, Norton L, Hudis CA (2016) Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol 34(10):1030–1033. https://doi.org/10.1200/JCO.2015.64.5515
Aseyev O, Stober C, Sulpher J, Clemons M, Johnson C, Fergusson D, Vandermeer L, Mazzarello S, Dent S (2017) Comparison of two care schedules for monitoring of cardiotoxicity in patients receiving trastuzumab-based therapy for early-stage breast cancer: study protocol for a randomized controlled non-inferiority trial. Clin Trials Degener Dis 2(2):40–45. https://doi.org/10.4103/2542-3975.209686
Funding
This research is supported by the National Health and Medical Research Council Centre of Research Excellence in Medicines Intelligence (ID: 1196900). MT is supported by an Australian Government Research Training Program Scholarship, a National Health and Medical Research Council Postgraduate Research Scholarship (ID: 1151479), a National Breast Cancer Foundation Postgraduate Scholarship Top-Up (ID: DS-18–01), and a Translational Cancer Research Network Clinical PhD Scholarship Top-Up award, supported by the Cancer Institute NSW. AS is supported by a National Health and Medical Research Council Early Career Fellowship (ID: 1158763).
Author information
Authors and Affiliations
Contributions
MT, AS and SAP were responsible for conceiving of and designing the study. MT and AS were responsible for statistical analysis. MT, AS, BEK, BD, RJS, CKL and SAP were responsible for data analysis and interpretation. MT was responsible for writing the manuscript. MT, AS, BEK, BD, RJS, CKL and SAP were responsible for editing and reviewing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Belinda E. Kiely reports funding from Roche and TEVA for participating in advisory boards; speakers fees from Roche and Novartis; and travel/conference expenses from Roche. Chee K. Lee reports grants from AstraZeneca and personal fees from AstraZeneca, Novartis, Pfizer, Roche, Boehringer Ingelhein, Takeda, outside of the submitted work. Sallie-Anne Pearson is a member of the Drug Utilisation Sub Committee of the Pharmaceutical Benefits Advisory Committee. The views expressed in this paper do not represent those of the Committee. The Centre for Big Data Research in Health, UNSW Sydney has received funding from AbbVie Australia to conduct research, unrelated to the present study. AbbVie did not have any knowledge of, or involvement in, the present study. All remaining authors have declared no conflicts of interest relevant to the submitted work.
Ethical approval
This study was approved by the New South Wales Population and Health Services Research Ethics Committee (Approval Number: 2010/02/213). The Ethics Committee granted a waiver of the usual requirement for the consent of the individual to the use of their health information in a research project, in line with the Guidelines approved under Sect. 95/95A of the Privacy Act 1988. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, M., Schaffer, A.L., Kiely, B.E. et al. Cardiac assessment in Australian patients receiving (neo)adjuvant trastuzumab for HER2-positive early breast cancer: a population-based study. Breast Cancer Res Treat 187, 893–902 (2021). https://doi.org/10.1007/s10549-021-06135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06135-5