Abstract
Purpose
Human epidermal growth factor-receptor-2 (HER2) is a membrane-tyrosine-kinase that is amplified/overexpressed up to 20% in breast cancer. HER2 positive status is associated with faster disease progression, higher metastatic potential, and shorter disease-free/overall survival and also has emerged as an important therapeutic target in breast cancer. HER2 status can be determined by in-situ-hybridization (ISH) or immunohistochemistry (IHC). Although the concordance rate between ISH and IHC is well-known, the prognostic power of both technologies if tested in parallel on the same tumor has not been studied extensively.
Methods
In this study we retrospectively analyzed a large HER2 positive breast cancer cohort tested both with fluorescence labeled ISH (FISH) and IHC in parallel on each case. We stratified HER2 positive results by FISH and IHC with long-term overall survival, 5-year survival and metastases/recurrence rates. Positive HER2 status both FISH and IHC was available in 364 patients.
Results
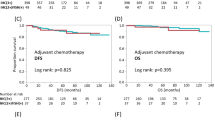
The number of HER2 FISH-positive and FISH-negative patients was 342 and 22, respectively. The number of HER2 IHC 0/1 + , IHC 2 + , and IHC 3 + patients was 12, 42, and 310, respectively. Among the patients with IHC 3 + status, 288 were FISH-positive and 22 FISH-negative. HER2 status determined by FISH correlated with clinical outcomes (overall survival and with metastases/recurrence, p = 0.036, p = 0.039), whereas HER2 status determined by IHC did not.
Conclusion
Our results indicate that prognostic information in HER2 positive breast cancer also depends on the methodology of how positivity was determined. In our cohort, FISH was superior to IHC based positive HER2 status.


Similar content being viewed by others
References
Adamczyk A, Kruczak A, Harazin-Lechowska A, Ambicka A, Grela-Wojewoda A, Domagala-Haduch M, Janecka-Widla A, Majchrzyk K, Cichocka A, Rys J, Niemiec J (2018) Relationship between HER2 gene status and selected potential biological features related to trastuzumab resistance and its influence on survival of breast cancer patients undergoing trastuzumab adjuvant treatment. Onco Targets Ther 11:4525–4535. https://doi.org/10.2147/OTT.S166983
Ahmed S, Sami A, Xiang J (2015) HER2-directed therapy: current treatment options for HER2-positive breast cancer. Breast Cancer 22:101–116. https://doi.org/10.1007/s12282-015-0587-x
Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM, Group CS (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366:109–119. https://doi.org/10.1056/NEJMoa1113216
Bilous M, Dowsett M, Hanna W, Isola J, Lebeau A, Moreno A, Penault-Llorca F, Ruschoff J, Tomasic G, van de Vijver M (2003) Current perspectives on HER2 testing: a review of national testing guidelines. Mod Pathol 16:173–182. https://doi.org/10.1097/01.MP.0000052102.90815.82
Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G Jr, Untch M, Smith I, Gianni L, Baselga J, Al-Sakaff N, Lauer S, McFadden E, Leyland-Jones B, Bell R, Dowsett M, Jackisch C (2017) 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 389:1195–1205. https://doi.org/10.1016/S0140-6736(16)32616-2
Dowsett M, Procter M, McCaskill-Stevens W, de Azambuja E, Dafni U, Rueschoff J, Jordan B, Dolci S, Abramovitz M, Stoss O, Viale G, Gelber RD, Piccart-Gebhart M, Leyland-Jones B (2009) Disease-free survival according to degree of HER2 amplification for patients treated with adjuvant chemotherapy with or without 1 year of trastuzumab: the HERA Trial. J Clin Oncol 27:2962–2969. https://doi.org/10.1200/JCO.2008.19.7939
Dressler LG, Berry DA, Broadwater G, Cowan D, Cox K, Griffin S, Miller A, Tse J, Novotny D, Persons DL, Barcos M, Henderson IC, Liu ET, Thor A, Budman D, Muss H, Norton L, Hayes DF (2005) Comparison of HER2 status by fluorescence in situ hybridization and immunohistochemistry to predict benefit from dose escalation of adjuvant doxorubicin-based therapy in node-positive breast cancer patients. J Clin Oncol 23:4287–4297. https://doi.org/10.1200/JCO.2005.11.012
Gancberg D, Di Leo A, Cardoso F, Rouas G, Pedrocchi M, Paesmans M, Verhest A, Bernard-Marty C, Piccart MJ, Larsimont D (2002) Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol 13:1036–1043. https://doi.org/10.1093/annonc/mdf252
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743. https://doi.org/10.1056/NEJMoa064320
Gown AM, Goldstein LC, Barry TS, Kussick SJ, Kandalaft PL, Kim PM, Tse CC (2008) High concordance between immunohistochemistry and fluorescence in situ hybridization testing for HER2 status in breast cancer requires a normalized IHC scoring system. Mod Pathol 21:1271–1277. https://doi.org/10.1038/modpathol.2008.83
Gullo G, Bettio D, Zuradelli M, Masci G, Giordano L, Bareggi C, Tomirotti M, Salvini P, Runza L, La Verde N, Santoro A (2013) Level of HER2/neu amplification in primary tumours and metastases in HER2-positive breast cancer and survival after trastuzumab therapy. Breast 22:190–193. https://doi.org/10.1016/j.breast.2013.01.005
Hicks DG, Tubbs RR (2005) Assessment of the HER2 status in breast cancer by fluorescence in situ hybridization: a technical review with interpretive guidelines. Hum Pathol 36:250–261. https://doi.org/10.1016/j.humpath.2004.11.010
Krishnamurti U, Silverman JF (2014) HER2 in breast cancer: a review and update. Adv Anat Pathol 21:100–107. https://doi.org/10.1097/PAP.0000000000000015
Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, Leiberman G, Slamon DJ (2005) Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer 6:240–246. https://doi.org/10.3816/CBC.2005.n.026
Middleton LP, Price KM, Puig P, Heydon LJ, Tarco E, Sneige N, Barr K, Deavers MT (2009) Implementation of American Society of Clinical Oncology/College of American Pathologists HER2 Guideline Recommendations in a tertiary care facility increases HER2 immunohistochemistry and fluorescence in situ hybridization concordance and decreases the number of inconclusive cases. Arch Pathol Lab Med 133:775–780. https://doi.org/10.1043/1543-2165-133.5.775
Moatamed NA, Nanjangud G, Pucci R, Lowe A, Shintaku IP, Shapourifar-Tehrani S, Rao N, Lu DY, Apple SK (2011) Effect of ischemic time, fixation time, and fixative type on HER2/neu immunohistochemical and fluorescence in situ hybridization results in breast cancer. Am J Clin Pathol 136:754–761. https://doi.org/10.1309/AJCP99WZGBPKCXOQ
Moelans CB, de Weger RA, Van der Wall E, van Diest PJ (2011) Current technologies for HER2 testing in breast cancer. Crit Rev Oncol Hematol 80:380–392. https://doi.org/10.1016/j.critrevonc.2010.12.005
Perez EA, Reinholz MM, Hillman DW, Tenner KS, Schroeder MJ, Davidson NE, Martino S, Sledge GW, Harris LN, Gralow JR, Dueck AC, Ketterling RP, Ingle JN, Lingle WL, Kaufman PA, Visscher DW, Jenkins RB (2010) HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol 28:4307–4315. https://doi.org/10.1200/JCO.2009.26.2154
Press MF, Sauter G, Buyse M, Fourmanoir H, Quinaux E, Tsao-Wei DD, Eiermann W, Robert N, Pienkowski T, Crown J, Martin M, Valero V, Mackey JR, Bee V, Ma Y, Villalobos I, Campeau A, Mirlacher M, Lindsay MA, Slamon DJ (2016) HER2 gene amplification testing by fluorescent in situ hybridization (FISH): comparison of the ASCO-college of american pathologists guidelines with FISH scores used for enrollment in breast cancer international research group clinical trials. J Clin Oncol 34:3518–3528. https://doi.org/10.1200/JCO.2016.66.6693
Ross JS, Fletcher JA (1998) The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Oncologist 3:237–252
Sapino A, Maletta F, Verdun di Cantogno L, Macri L, Botta C, Gugliotta P, Scalzo MS, Annaratone L, Balmativola D, Pietribiasi F, Bernardi P, Arisio R, Viberti L, Guzzetti S, Orlassino R, Ercolani C, Mottolese M, Viale G, Marchio C (2014) Gene status in HER2 equivocal breast carcinomas: impact of distinct recommendations and contribution of a polymerase chain reaction-based method. Oncologist 19:1118–1126. https://doi.org/10.1634/theoncologist.2014-0195
Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF (2009) Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol 27:1323–1333. https://doi.org/10.1200/JCO.2007.14.8197
Shui R, Liang X, Li X, Liu Y, Li H, Xu E, Zhang Z, Lian Y, Guo S, Yao M, Yang H, Xu F, Liu Y, Liu J, Guo D, Wang K, Li J, Ma Y, Wang J, Shi J, Bu H, Yang W (2020) Hormone receptor and human epidermal growth factor receptor 2 detection in invasive breast carcinoma: a retrospective study of 12,467 patients from 19 chinese representative clinical centers. Clin Breast Cancer 20:e65–e74. https://doi.org/10.1016/j.clbc.2019.07.013
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792. https://doi.org/10.1056/NEJM200103153441101
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J, Breast Cancer International Research G (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273–1283. https://doi.org/10.1056/NEJMoa0910383
Stocker A, Hilbers ML, Gauthier C, Grogg J, Kullak-Ublick GA, Seifert B, Varga Z, Trojan A (2016) HER2/CEP17 ratios and clinical outcome in HER2-positive early breast cancer undergoing trastuzumab-containing therapy. PLoS ONE 11:e0159176. https://doi.org/10.1371/journal.pone.0159176
Torre LA, Siegel RL, Ward EM, Jemal A (2016) Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomark Prev 25:16–27. https://doi.org/10.1158/1055-9965.EPI-15-0578
Tubbs RR, Hicks DG, Cook J, Downs-Kelly E, Pettay J, Hartke MB, Hood L, Neelon R, Myles J, Budd GT, Moore HC, Andresen S, Crowe JP (2007) Fluorescence in situ hybridization (FISH) as primary methodology for the assessment of HER2 Status in adenocarcinoma of the breast: a single institution experience. Diagn Mol Pathol 16:207–210. https://doi.org/10.1097/PDM.0b013e318064c72a
Varga Z, Noske A (2015) Impact of modified 2013 ASCO/CAP guidelines on HER2 testing in breast cancer One Year Experience. PLoS ONE 10:e0140652. https://doi.org/10.1371/journal.pone.0140652
Varga Z, Noske A, Ramach C, Padberg B, Moch H (2013) Assessment of HER2 status in breast cancer: overall positivity rate and accuracy by fluorescence in situ hybridization and immunohistochemistry in a single institution over 12 years: a quality control study. BMC Cancer 13:615. https://doi.org/10.1186/1471-2407-13-615
Varga Z, Tubbs RR, Moch H (2014) Concomitant detection of HER2 protein and gene alterations by immunohistochemistry (IHC) and silver enhanced in situ hybridization (SISH) identifies HER2 positive breast cancer with and without gene amplification. PLoS ONE 9:e105961. https://doi.org/10.1371/journal.pone.0105961
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K, Group ES (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783–1791. https://doi.org/10.1056/NEJMoa1209124
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF, American Society of Clinical O, College of American P (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145. https://doi.org/10.1200/JCO.2006.09.2775
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human Epidermal growth factor receptor 2 testing in breast cancer: American Society of clinical oncology/college of American pathologists clinical practice guideline focused update. Arch Pathol Lab Med 142:1364–1382. https://doi.org/10.5858/arpa.2018-0902-SA
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of clinical oncology/college of american pathologists clinical practice guideline focused update. J Clin Oncol 36:2105–2122. https://doi.org/10.1200/JCO.2018.77.8738
Yamashita H, Ishida N, Hatanaka Y, Hagio K, Oshino T, Takeshita T, Kanno-Okada H, Shimizu AI, Hatanaka KC, Matsuno Y (2020) HER2 gene amplification in ER-positive HER2 immunohistochemistry 0 or 1+ Breast cancer with early recurrence. Anticancer Res 40:645–652. https://doi.org/10.21873/anticanres.13994
Zabaglo L, Stoss O, Ruschoff J, Zielinski D, Salter J, Arfi M, Bradbury I, Dafni U, Piccart-Gebhart M, Procter M, Dowsett M, Team HTS (2013) HER2 staining intensity in HER2-positive disease: relationship with FISH amplification and clinical outcome in the HERA trial of adjuvant trastuzumab. Ann Oncol 24:2761–2766. https://doi.org/10.1093/annonc/mdt275
Funding
No funding was necessary for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ZV received consultancy and speaking fees from Roche, Astra Zeneca and Genomic Health. There is no conflict of interest to disclose from the other authors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical permission for the project was granted by the Ethical Commission of the Canton Zurich (No KEK-ZH-2012-553). Patients provided written or oral-informed consent for participation in the study at the time of data retrieval or during follow-up consultations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stocker, A., Trojan, A., Elfgen, C. et al. Differential prognostic value of positive HER2 status determined by immunohistochemistry or fluorescence in situ hybridization in breast cancer. Breast Cancer Res Treat 183, 311–319 (2020). https://doi.org/10.1007/s10549-020-05772-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05772-6