Abstract
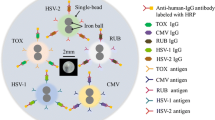
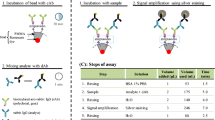
An automated microfluidic system with functionalized beads has been developed for multiplexed TORCH detection at point-of-care testing. A concise microfluidic chip consisting of a one-dimensional beads array is developed to simultaneously detect TOX, RUB, CMV, HSV-I and HSV-II respectively with five functionalized beads. A compact liquid handling module has been developed to automate the sandwiched chemiluminescence immunoassay within the one-dimensional beads array of the microfluidic chip. A precise ram pump is adopted to not only add reagent into the microfluidic chip from outside, but also facilitate elaborate fluid control inside the microfluidic chip for improved performance. A large-size waste chamber with a liquid-absorbing sponge holds the waste reagent within the microfluidic chip to prevent backflow. The one-dimensional beads array is heated from double-sides at 37 ℃ for sensitive detection with reduced time. A sensitive CMOS camera is adopted to take chemiluminescence image from the one-dimensional beads array, and a custom processing algorithm is adopted to analyze the image. For each serum sample, five different infections can be simultaneously detected with the automated microfluidic system. Experimental results show that efficient, sensitive, and accurate multiplexed TORCH detection can be conveniently achieved with the integrated microfluidic system.






Similar content being viewed by others
References
M. Barbi, S. Binda, S. Caroppo, V. Primache, Neonatal screening for congenital cytomegalovirus infection and hearing loss. J. Clin. Virol. 35, 206–209 (2006)
N. Chang, J. Zhai, B. Liu, J. Zhou, Z. Zeng, X. Zhao, Low cost 3D microfluidic chips for multiplex protein detection based on photonic crystal beads. Lab. Chip 18, 3638–3644 (2018)
D. Chen, M. Mauk, X. Qiu, C. Liu, J. Kim, S. Ramprasad, S. Ongagna, W.R. Abrams, D. Malamud, P.L.A.M. Corstjens, H.H. Bau, An integrated microfluidic cassette for isolation, amplification, and detection of nucleic acids. Biomed. Microdevices 12(4), 705–719 (2010)
C.Y. Chung, J.C. Wang, H.S. Chuang, Rapid Bead-Based Antimicrobial Susceptibility Testing by Optical Diffusometry. PLoS One 11, e0148864 (2016)
H. Ji, L. Chang, J. Zhao, L. Zhang, X. Jiang, F. Guo, L. Wang, Evaluation of ELISA and CLIA for Treponema pallidum specific antibody detection in China: A multicenter study. J. Microbiol. Methods 166, 105742 (2019)
L. Jiang, Z. Yu, W. Du, Z. Tang, T. Jiang, C. Zhang, Z. Lu, Development of a fluorescent and colorimetric detection methods-based protein microarray for serodiagnosis of TORCH infections. Biosens. Bioelectron. 24, 376–382 (2008)
E.P.D. Jong, A.C.T.M. Vossen, F.J. Walther, E. Lopriore, How to use… neonatal TORCH testing. Arch. Dis. Child. Educ. Pract. Ed. 98, 93 (2013)
D. Li, H. Yang, W.H. Zhang, H. Pan, D.Q. Wen, F.C. Han, H.F. Guo, X.M. Wang, X.J. Yan, A simple parallel analytical method of prenatal screening. Gynecol. Obstet. Invest. 62, 220–225 (2006)
C.T. Lim, Y. Zhang, Bead-based microfluidic immunoassays: the next generation. Biosens. Bioelectron. 22, 1197–1204 (2007)
C. Liu, X. Qiu, S. Ongagna, D. Chen, Z. Chen, W.R. Abrams, D. Malamud, P.L.A.M. Corstjens, H.H. Bau, A timer-actuated immunoassay cassette for detecting molecular markers in oral fluids. Lab. Chip 9, 768–776 (2009)
O. Mayborodaa, I. Katakisa, C. K. O’Sullivana, Multiplexed isothermal nucleic acid amplification. Anal. Biochem. 545, 20–30 (2018)
N. Neu, J. Duchon, P. Zachariah, TORCH infections. Clin. Perinatol. 42, 77–103 (2015)
T. Nguyen, V.A. Chidambara, S.Z. Andreasen, M. Golabi, A. Wolff, Point-of-care devices for pathogen detections: the three most important factors to realize towards commercialization. Trends Analyt. Chem. 131, 116004 (2020)
X. Qiu, J.A. Thompson, Z. Chen, C. Liu, D. Chen, S. Ramprasad, M.G. Mauk, S. Ongagna, C. Barber, W.R. Abrams, D. Malamud, P.L.A.M. Corstjens, H.H. Bau, Finger-actuated, self-contained immunoassay cassettes. Biomed. Microdevices 11, 1175–1186 (2009)
X. Qiu, S. Yang, D. Wu, D. Wang, S. Qiao, S. Ge, N. Xia, D. Yu, S. Qian, Rapid enumeration of CD4 + T lymphocytes using an integrated microfluidic system based on Chemiluminescence image detection at point-of-care testing. Biomed. Microdevices 20(1), 15 (2018)
X. Qiu, J. Zhang, S. Gong, D. Wang, S. Qiao, S. Ge, N. Xia, D. Yu, S. Qian, A Single-Bead-Based, Fully Integrated Microfluidic System for High-Throughput CD4 + T Lymphocyte Enumeration. SLAS Technol. 23(2), 134–143 (2017)
J.H. Seo, B.H. Park, S.J. Oh, G. Choi, D.H. Kim, E.Y. Lee, T.S. Seo, Development of a high-throughput centrifugal loop-mediated isothermal amplification microdevice for multiplex foodborne pathogenic bacteria detection. Sens. Actuators B Chem. 246, 146–153 (2017)
J. Song, C. Liu, M.G. Mauk, S.C. Rankin, J.B. Lok, R.M. Greenberg, H.H. Bau, Two-stage isothermal enzymatic amplification for concurrent multiplex molecular detection. Clin. Chem. 63(3), 714–722 (2017)
C. Wang, M. Liu, Z. Wang, S. Li, Y. Deng, N. He, Point-of-care diagnostics for infectious diseases: from methods to devices. Nano Today 37, 101092 (2021)
H. Yang, Q. Guo, R. He, D. Li, X. Zhang, C. Bao, H. Hu, D. Cui, A Quick and Parallel Analytical Method Based on Quantum Dots Labeling for ToRCH-Related Antibodies. Nanoscale Res. Lett. 4, 1469–1474 (2009)
H. Zhang, L. Liu, X. Fu, Z. Zhu, Microfluidic beads-based immunosensor for sensitive detection of cancer biomarker proteins using multienzyme-nanoparticle amplification and quantum dots labels. Biosens. Bioelectron. 42, 23–30 (2013)
Q. Zhang, S. Feng, L. Lin, S. Mao, J. Lin, Emerging open microfluidics for cell manipulation. Chem. Soc. Rev. 50, 5333–5348 (2021)
K. Zheng, C. Chen, X. Chen, M. Xu, L. Chen, Y. Hu, Y. Bai, B. Liu, C. Yan, H. Wang, J. Li, Graphically encoded suspension array for multiplex immunoassay and quantification of autoimmune biomarkers in patient sera. Biosens. Bioelectron. 132, 47–54 (2019)
J. Zhuang, J. Yin, S. Lv, B. Wang, Y. Mu, Advanced “lab-on-a-chip” to detect viruses – Current challenges and future perspectives. Biosens. Bioelectron. 163, 112291 (2020)
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 81871505, 61971026), the Fundamental Research Fund for the Central Universities (XK1802-4), the National Science and Technology Major Project (2018ZX10732101-001-009), and the research fund to the top scientific and technological innovation team from Beijing University of Chemical Technology (No. buctylkjcx06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Yu, S., Wang, D. et al. An automated microfluidic system with one-dimensional beads array for multiplexed torch detection at point-of-care testing. Biomed Microdevices 24, 38 (2022). https://doi.org/10.1007/s10544-022-00629-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s10544-022-00629-9