Abstract
Background
Diphtheria can be prevented by vaccination, but some epidemics occur in several places, and diphtheria’s threat is considerable. Administration of diphtheria antitoxin (DAT) produced from hyperimmunized animals is the most common treatment. Recombinant human antibody fragments such as single-chain variable fragments (scFv) produced by phage display library may introduce an interesting approach to overcome the limitations of the traditional antibody therapy. In the present study, B cells of immunized volunteers were used to construct a human single-chain fragment (HuscFv) library.
Materials and methods
The library was constructed with the maximum combination of heavy and light chains. As an antigen, Diphtheria toxoid (DTd) was used in four-round phage bio-panning to select phage clones that display DTd bound HuscFv from the library. After panning, individual scFv clones were selected. Clones that were able to detect DTd in an initial screening assay were transferred to Escherichia coli HB2151 to express the scFvs and purification was followed by Ni metal ion affinity chromatography. Toxin neutralization test was performed on Vero cells. The reactivity of the soluble scFv with diphtheria toxin were done and affinity calculation based on Beatty method was calculated.
Results
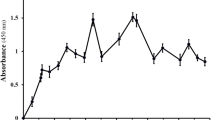
The size of the constructed scFv library was calculated to be 1.3 × 106 members. Following four rounds of selection, 40 antibody clones were isolated which showed positive reactivity with DTd in an ELISA assay. Five clones were able to neutralize DTd in Vero cell assay. These neutralizing clones were used for soluble expression and purification of scFv fragments. Some of these soluble scFv fragments show neutralizing activity ranging from 0.6 to 1.2 µg against twofold cytotoxic dose of diphtheria toxin. The affinity constant of the selected scFv antibody was determined almost 107 M−1.
Conclusion
This study describes the prosperous construction and isolation of scFv from the immune library, which specifically neutralizes diphtheria toxin. The HuscFv produced in this study can be a potential candidate to substitute the animal antibody for treating diphtheria and detecting toxins.







Similar content being viewed by others
Data availability
The data supporting the findings of the article is available within the article.
References
Alvarenga LM et al (2014) Engineering venom’s toxin-neutralizing antibody fragments and its therapeutic potential. Toxins 6(8):2541–2567
Beatty JD, Beatty BG, Vlahos WG (1987) Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Methods 100(1–2):173–179
Bissumbhar B et al (2004) Evaluation of diphtheria convalescent patients to serve as donors for the production of anti-diphtheria immunoglobulin preparations. Vaccine 22(15):1886–1891
Both L et al (2014) Access to diphtheria antitoxin for therapy and diagnostics. Eurosurveillance 19(24):20830
Danelli MdGM et al (1991) Protective monoclonal antibodies to diphtheria toxin. Mem Inst Oswaldo Cruz 86(2):265–267
Gao J et al (2010) Phage display and its application in vaccine design. Ann Microbiol 60(1):13–19
Gupta R, Siber G (1995) Use of in vitro Vero cell assay and ELISA in the United States potency test of vaccines containing adsorbed diphtheria and tetanus toxoids. Dev Biol Stand 86:207–215
Indrawattana N et al (2010) Human monoclonal ScFv that inhibits cellular entry and metalloprotease activity of tetanus neurotoxin. Asian Pac J Allergy Immunol 28(1):85–93
Jain A et al (2016) Diphtheria: it is still prevalent!!! Int J Pediatr Otorhinolaryngol 86:68–71
Kakita M et al (2006) Isolation of a human monoclonal antibody with strong neutralizing activity against diphtheria toxin. Infect Immun 74(6):3682–3683
Kang DG, Choi SS, Cha HJ (2006) Enhanced biodegradation of toxic organophosphate compounds using recombinant Escherichia coli with sec pathway-driven periplasmic secretion of organophosphorus hydrolase. Biotechnol Prog 22(2):406–410
Khalili E et al (2015) Production of recombinant human scFv against tetanus toxin heavy chain by phage display technology. Monoclon Antib Immunodiagn Immunother 34(5):303–309
Lakzaei M et al (2019) A comparison of three strategies for biopanning of phage-scFv library against diphtheria toxin. J Cell Physiol 234(6):9486–9494
Leka O et al (2014) Diphtheria toxin conformational switching at acidic pH. FEBS J 281(9):2115–2122
Miethe S et al (2015) Development of human-like scFv-Fc neutralizing botulinum neurotoxin E. PLoS ONE 10(10):e0139905
Mokrousov I (2009) Corynebacterium diphtheriae: genome diversity, population structure and genotyping perspectives. Infect Genet Evol 9(1):1–15
Murphy JR (1996) Corynebacterium diphtheriae. In: Baron S (ed) Medical microbiology. Galveston, TX, University of Texas Medical Branch at Galveston
Neelakantam B et al (2014) Recombinant human antibody fragment against tetanus toxoid produced by phage display. Eur J Microbiol Immunol 4(1):45–55
Newcombe C, Newcombe AR (2007) Antibody production: polyclonal-derived biotherapeutics. J Chromatogr B 848(1):2–7
Paschke M (2006) Phage display systems and their applications. Appl Microbiol Biotechnol 70(1):2–11
Pelat T et al (2007) High-affinity, human antibody-like antibody fragment (single-chain variable fragment) neutralizing the lethal factor (LF) of Bacillus anthracis by inhibiting protective antigen-LF complex formation. Antimicrob Agents Chemother 51(8):2758–2764
Phalipon A, Kaczorek M (1987) Genetically engineered diphtheria toxin fusion proteins carrying the hepatitis B surface antigen. Gene 55(2):255–263
Riches-Duit R et al (2021) Characterisation of diphtheria monoclonal antibodies as a first step towards the development of an in vitro vaccine potency immunoassay. Biologicals 69:38–48
Riegel P et al (1995) Taxonomy of Corynebacterium diphtheriae and related taxa, with recognition of Corynebacterium ulcerans sp. nov. nom. rev. FEMS Microbiol Lett 126(3):271–276
Schirrmann T, Hust M (2010) Construction of human antibody gene libraries and selection of antibodies by phage display. Immunotherapy of cancer. Springer, pp 177–209
Schirrmann T et al (2011) Phage display for the generation of antibodies for proteome research, diagnostics and therapy. Molecules 16(1):412–426
Sevigny LM et al (2013) Identification of a human monoclonal antibody to replace equine diphtheria antitoxin for treatment of diphtheria intoxication. Infect Immun 81(11):3992–4000
Smith J, Schallibaum EM (1970) The therapeutic effect of homologous and heterologous antitoxins in experimental diphtheria and tetanus. Br J Exp Pathol 51(1):73
Smith HL et al (2016) Potency of a human monoclonal antibody to diphtheria toxin relative to equine diphtheria anti-toxin in a guinea pig intoxication model. Virulence 7(6):660–668
Tohidkia MR et al (2012) Molecular considerations for development of phage antibody libraries. J Drug Target 20(3):195–208
Unkauf T et al (2016) Generation of recombinant antibodies against toxins and viruses by phage display for diagnostics and therapy. Protein targeting compounds. Springer, pp 55–76
Wagner K et al (2009) A review of the international issues surrounding the availability and demand for diphtheria antitoxin for therapeutic use. Vaccine 28(1):14–20
Wagner KS et al (2012) Diphtheria in the postepidemic period, Europe, 2000–2009. Emerg Infect Dis 18(2):217
Wenzel EV et al (2020) Human antibodies neutralizing diphtheria toxin in vitro and in vivo. Sci Rep 10(1):1–21
Zakikhany K, Efstratiou A (2012) Diphtheria in Europe: current problems and new challenges. Future Microbiol 7(5):595–607
Acknowledgements
Declared none.
Funding
This work was supported by Grant # 27676 from the Deputy of Research, Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
MA: concept, ideas, supervising and interpreting results. EK and ML: performed the experiments, wrote paper. All authors have read and agree with this paper.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest, financial or otherwise.
Ethics approval and consent to participate
Informed consent has been obtained from the participants involved. This study was approved by the Human Research Ethics Committee of Tehran University of Medical Sciences, Iran (IR. TUMS.MEDICINE.REC1395-27676).
Human and animal rights
No animals were used for studies that are the basis of this research. The reported experiments on humans are in accordance with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalili, E., Lakzaei, M. & Aminian, M. Neutralizing anti-diphtheria toxin scFv produced by phage display. Biotechnol Lett 46, 385–398 (2024). https://doi.org/10.1007/s10529-024-03476-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-024-03476-1