Abstract
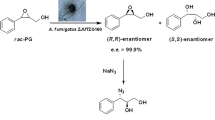
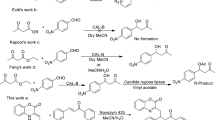
An effective preparation scheme for optically-active 3-pyrrolidinol and its derivatives based on biological transformation is proposed. Aspergillus sp. NBRC 109513 hydroxylated 1-benzoylpyrrolidine, yielding (S)-1-benzoyl-3-pyrrolidinol with 66 % ee. Kinetic resolution of 1-benzoyl-3-pyrrolidinol by Amano PS-IM lipase formed optically-active 1-benzoyl-3-pyrrolidinol with >99 % ee. (S)-1-Benzoyl-3-pyrrolidinol was successfully converted to 3-pyrrolidinol and its derivatives with by chemical reactions (>99 % ee).




Similar content being viewed by others
References
Brown HC, Vara Prasad JVM, Gupta AK (1986) Hydroboration. 78. Reinvestigation of the hydroboration of N-substituted 3-pyrrolines. Preparation of N-benzyl-3-pyrrolidinol and N-benzyl-3-pyrrolidinylborate of very high enantiomeric purity. J Org Chem 51:4296–4298
Hashimoto M, Eda Y, Osanai Y, Iwai T, Aoki S (1986) A novel decarboxylation of α-amino acids. A facile method of decarboxylation by the use of 2-cyclohexen-1-one as a catalyst. Chem Lett 1986:893–896
Inoue K, Kutsuki H, Hasegawa J, Takahashi S (1989) Process for preparing 3-pyrrolidinol. European Patent 0347818
Li Z, Feiten HJ, van Beilen JB, Duetz W, Witholt B (1999) Preparation of optically-active N-benzyl-3-hydroxypyrrolidine by enzymatic hydroxylation. Tetrahedron Asymmetry 10:1323–1333
Li Z, Feiten HJ, Chang D, Duetz W, Beilen JB, Witholt B (2001) Preparation of (R)- and (S)-N-protected 3-hydroxypyrrolidines by hydroxylation with Sphingomonas sp. HXN-200, a highly active, region- and stereoselective, and easy to handle biocatalyst. J Org Chem 66:8424–8430
Miyadera T, Sugimura Y, Hashimoto T, Tanaka T, Iino K, Shibata T, Sugawara S (1983) Synthesis and vitro activity of a new carbapenem, RS-533. J Antibiot 36:1034–1039
Nagahara T, Yokoyama Y, Inamura K, Katakura S, Komoriya S, Yamaguchi H, Hara T, Iwamoto M (1994) Dibasic (amidinoaryl) propanoic acid derivatives as novel blood coagulation factor Xa inhibitors. J Med Chem 37:1200–1207
Narita H, Konishi Y, Nitta J, Nagaki H, Kobayashi Y, Watanabe Y, Minami S, Saikawa I (1986) Pyridonecarboxylic acid as a antibacterial agents. IV. Synthesis and structure–activity relationship of 7-amino-1-aryl-6-fluoro-4-quinolone-3-carboxylic acids. Yakugaku Zassi 106:795–801
Parshikov IA, Modyanova LV, Dovgilivich EV, Terent’ev PB, Vorob’eva LI, Grishina GV (1992) Microbiological transformation of nitrogen-containing heterocyclic compounds. 3. Microbiological synthesis of hydroxyl derivatives of 1-benzoylpiperidine and 1-benzoylpyrrolidine. Chem Heterocycl Compd 28:159–162
Seido N, Okeda Y, Kumobayashi H (1991) Process for preparing optical active 3-hydroxypyrrolidine. European Patent 0452143
Tamazawa K, Arima H, Kojima T, Isomura Y, Okada M, Fujita S, Furuya T, Takenaka T, Inagaki O, Terai M (1986) Stereoselectivity of a potent calcium antagonist, 1-benzyl-3-pyrrolidinyl methyl 2,6-dimethyl-4-(m-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate. J Med Chem 29:2504–2511
Tomori H, Shibutani K, Ogura K (1996) Lipase-catalyzed practical synthesis of (R)-1-benzyl-3-hydroxy-2,5-pyrrolidinedione and related compounds. Bull Chem Soc Jpn 69:207–215
Acknowledgments
We would like to thank Dr. Mitsuhiko Fujiwara of Takasago International Corporation for his helpful discussions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yamada, S., Shimada, H., Yamada, R. et al. Preparation of optically-active 3-pyrrolidinol and its derivatives from 1-benzoylpyrrolidinol by hydroxylation with Aspergillus sp. and stereo-selective esterification by a commercial lipase. Biotechnol Lett 36, 595–600 (2014). https://doi.org/10.1007/s10529-013-1394-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1394-0