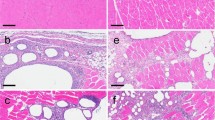
For antibody-based drugs, it is important and relevant to study their toxic effects, which can often become limiting when prescribing this type of therapy. General toxicity of antiviral drug Ergoferon based on technologically processed antibodies was studied on sexually mature animals. Analysis of acute toxicity showed the absence of lethal outcomes when the drug was administered to adult rats at the maximum tolerated doses. In a study of repeated dose toxicity, no adverse effects of the drug were detected.
Similar content being viewed by others
References
Wang Z, Wang G, Lu H, Li H, Tang M, Tong A. Development of therapeutic antibodies for the treatment of diseases. Mol. Biomed. 2022;3(1):35. doi: https://doi.org/10.1186/s43556-022-00100-4
Pelegrin M, Naranjo-Gomez M, Piechaczyk M. Antiviral monoclonal antibodies: can they be more than simple neutralizing agents? Trends Microbiol. 2015;23(10):653-665. doi: https://doi.org/10.1016/j.tim.2015.07.005
Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010;9(4):325-338. doi: https://doi.org/10.1038/nrd3003
Mokhtary P, Pourhashem Z, Mehrizi AA, Sala C, Rappuoli R. Recent progress in the discovery and development of monoclonal antibodies against viral infections. Biomedicines. 2022;10(8):1861. doi: https://doi.org/10.3390/biomedicines10081861.
Gudkov SV, Penkov NV, Baimler IV, Lyakhov GA, Pustovoy VI, Simakin AV, Sarimov RM, Scherbakov IA. Effect of mechanical shaking on the physicochemical properties of aqueous solutions. Int. J. Mol. Sci. 2020;21(21):8033. doi: https://doi.org/10.3390/ijms21218033
Gudkov SV, Baimler IV, Uvarov OV, Smirnova VV, Volkov MY, Semenova AA, Lisitsyn AB. Influence of the concentration of Fe and Cu nanoparticles on the dynamics of the size distribution of nanoparticles. Front. Phys. 2020;8:622551. doi: https://doi.org/10.3389/fphy.2020.622551
Ryzhkina IS, Murtazina LI, Kiseleva YV, Konovalov AI. Self-organization and physicochemical properties of aqueous solutions of the antibodies to interferon gamma at ultrahigh dilution. Dokl. Phys. Chem. 2015;462(1):110-114. doi: https://doi.org/10.1134/S0012501615050048
Tarasov SA, Gorbunov EA, Don ES, Emelyanova AG, Kovalchuk AL, Yanamala N, Schleker ASS, Klein-Seetharaman J, Groenestein R, Tafani JP, van der Meide P, Epstein OI. Insights into the mechanism of action of highly diluted biologics. J. Immunol. 2020;205(5):1345-1354. doi: https://doi.org/10.4049/jimmunol.2000098
Geppe NA, Blokhin BM, Shamsheva OV, Abdrakhmanova ST, Alikhanova KA, Myrzabekova GT. Efficacy and safety of Ergoferon in children from 6 months to 6 years old with acute respiratory viral infections in contemporary outpatient practice: A Multicenter, Double-Blind, Placebo-Controlled Randomized Trial. Can. Respir. J. 2021;2021:5570178. doi: https://doi.org/10.1155/2021/5570178
Gorelov AV, Geppe NA, Blokhin BM, Zaitsev AA, Usenko DV, Nikolaeva SV, Nikiforov VV, Skuchalina LN, Shamiyev FM. Impact of immunomodulation therapy on the course of acute viral respiratory infections: a meta-analysis of clinical trials assessing the efficacy and safety of Ergoferon in the treatment of influenza and other acute respiratory viral infections. Voprosy. Prakt. Pediatrii. 2021;16(4):83-97. Russian. doi: https://doi.org/10.20953/1817-7646-2021-4-83-97
Zhdanov KV, Khamitov RF, Rafalsky VV, Mikhaylusova MP, Shapovalova YuS, Oseshnyuk RA, Alpenidze DN. The use of antiviral drug based on technologically processed antibodies to interferon-7, CD4 receptor and histamine in the treatment of influenza in adults: results of a multicenter open-label randomized comparative trial with oseltamivir. Infekts. Bol. 2021;19(1):39-57. Russian. doi: https://doi.org/10.20953/1729-9225-2021-1-39-57
Kostinov MP, Khamitov RF, Babkin AP, Minina ES, Bart BYa, Mikhailusova MP, Yanovskaya ME, Sherenkov AO, Petrov DV, Alpenidze DN, Shapovalova YuS, Chernogorova MV, Pavlysh EF, Sardinov RT. Treatment of acute respiratory infection in adults: results of a multicenter randomized double-blind placebo-controlled clinical trial. Lech. Vrach. 2019;(10):72-79. Russian. doi: https://doi.org/10.26295/OS.2019.29.30.015
Emelianova AG, Tarasov SA, Morozov SG. Anti-inflammatory activity of released-active antibodies to interferon-gamma, CD4-receptor, and histamine against respiratory-syncytial viral infection. Patogenez. 2019;17(1):85-89. Russian. doi: https://doi.org/10.25557/2310-0435.2019.01.85-89
Arzamastsev EV, Berezovskaya IV, Verstakova OL, Gus’kova TA, Durnev AD, Ivanova AS, Krepkova LV, Sorokina AV. Methodical recommendations for the study of general toxic effects of drugs. Manual for Preclinical Studies of New Pharmacological Substances. Part I, Mironov AN, ed. Moscow, 2013. P. 13-24. Russian.
Epstein OI, Shtark MB, Kolyadko TM. Method of treating a pathological syndrome and a pharmaceutical agent. Patent US-2008050360-A1. Priority 2000/06/20.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 175, No. 5, pp. 581-585, May, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Danchenko, E.A., Ertuzun, I.A., Bugaeva, L.I. et al. Analysis of General Toxicity of Ergoferon. Bull Exp Biol Med 175, 644–648 (2023). https://doi.org/10.1007/s10517-023-05918-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-023-05918-8