Abstract
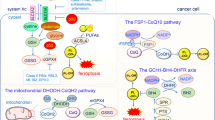
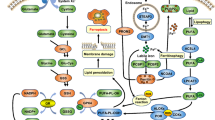
Ferroptosis is a new discovered regulated cell death triggered by the ferrous ion (Fe2+)-dependent accumulation of lipid peroxides associated with cancer and many other diseases. The mechanism of ferroptosis includes oxidation systems (such as enzymatic oxidation and free radical oxidation) and antioxidant systems (such as GSH/GPX4, CoQ10/FSP1, BH4/GCH1 and VKORC1L1/VK). Among them, ferroptosis suppressor protein 1 (FSP1), as a crucial regulatory factor in the antioxidant system, has shown a crucial role in ferroptosis. FSP1 has been well validated to ferroptosis in three ways, and a variety of intracellular factors and drug molecules can alleviate ferroptosis via FSP1, which has been demonstrated to alter the sensitivity and effectiveness of cancer therapies, including chemotherapy, radiotherapy, targeted therapy and immunotherapy. This review aims to provide important frameworks that, bring the regulation of FSP1 mediated ferroptosis into cancer therapies on the basis of existing studies.



Similar content being viewed by others
Abbreviations
- ACSL4:
-
Acyl-CoA synthetase long-chain family member 4
- AEC2s:
-
Alveolar epithelial Type2 cells
- AIFM2:
-
Apoptosis-inducing factor mitochondrial 2
- ALL:
-
Acute lymphoblastic leukemia
- ANXA7:
-
Annexin A7
- AR:
-
Androgen receptor
- BH4:
-
Tetrahydrobiopterin
- BMP:
-
BODIPY-modified polyamide
- CHMP5:
-
Charged multivesicular body protein 5
- CHMP6:
-
Charged multivesicular body protein 6
- CoQ10:
-
Ubiquinone-10
- CoQ10H2:
-
Ubiquinol
- CRPC:
-
Castration-resistant prostate cancer
- CTRP:
-
Cancer Therapeutics Response Portal
- CTD:
-
C-terminal domain
- CYP2J2:
-
Cytochrome P450 2J2
- DAMP:
-
Damage-associated molecular patterns
- DHFR:
-
Dihydrofolate reductase
- DHODH:
-
Dihydroorotate dehydrogenase
- EMT:
-
Epithelial-mesenchymal transformation
- ER:
-
Estrogen receptor
- ESCC:
-
Esophageal squamous cell carcinoma
- ETC:
-
Electronic transport chain
- FSEN1:
-
Ferroptosis sensitizer Ferroptosis sensitizer 1
- FSP1:
-
Ferroptosis suppressor protein 1
- GCH1:
-
GTP cyclohydrolase-1
- GPX4:
-
Glutathione peroxidase 4
- GSH:
-
Glutathione
- HA:
-
Hyaluronic acid
- HCC:
-
Hepatocellular carcinoma
- HMGB1:
-
High mobility group box 1
- ICD:
-
Immunogenic cell death
- IPP:
-
Isopentenyl pyrophosphate
- IR:
-
Ionizing radiation
- LPCAT3:
-
Lysophosphatidylcholine acyltransferase 3
- LSCs:
-
Lung cancer stem cells
- MDA:
-
Malondialdehyde
- MOF:
-
Metal-organic framework
- MK4:
-
Menaquinone-4
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PDCD6IP:
-
Programmed cell death 6-interacting protein
- PIE:
-
Propofol injectable emulsion
- PLOOH:
-
Phospholipid hydroperoxide
- PUFA:
-
Polyunsaturated fatty acid
- PUFA-CoA:
-
Coenzyme A-activated polyunsaturated fatty acid
- PUFA-PLs:
-
Polyunsaturated fatty acids phospholipids
- Py-RSL:
-
Pyridine RAS-selective lethal ligand
- QLD:
-
QiLing Decoction
- RCD:
-
Regulated cell death
- ROS:
-
Reactive oxygen species
- RT:
-
Radiotherapy
- RTA:
-
RTA Radical-trapping antioxidant
- TME:
-
Tumor microenvironment
- TSS:
-
Transcriptional start site
- TSG101:
-
Tumor susceptibility 101
- VKH2:
-
Vitamin K hydroquinone
- VK:
-
Vitamin K
- VKORC1L1:
-
Vitamin K epoxide reductase complex subunit 1 like 1
- 4HNE :
-
4-Hydroxynonenal
References
Galluzzi L et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25(3):486–541
Dixon SJ et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Li FJ et al (2022) System X(c) (-)/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front Pharmacol 13:910292
Bersuker K et al (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575(7784):688–692
Hu Q et al (2022) Blockade of GCH1/BH4 Axis Activates Ferritinophagy to Mitigate the Resistance of Colorectal Cancer to Erastin-Induced Ferroptosis. Front Cell Dev Biol 10:810327
Qiu B et al (2024) Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis. Cell 187(5):1177-1190.e18
Zhang F et al (2023) Current insights into the functional roles of ferroptosis in musculoskeletal diseases and therapeutic implications. Front Cell Dev Biol 11:1112751
Magtanong L et al (2019) Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem Biol 26(3):420-432.e9
Doll S et al (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13(1):91–98
Cui J et al (2023) LPCAT3 Is Transcriptionally Regulated by YAP/ZEB/EP300 and Collaborates with ACSL4 and YAP to Determine Ferroptosis Sensitivity. Antioxid Redox Signal 39(7–9):491–511
Lee H et al (2020) Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol 22(2):225–234
Wang K et al (2021) Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells. Cell Death Differ 28(4):1222–1236
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22(4):266–282
Ayala A, Munoz MF, Arguelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:360438
Conrad M, Pratt DA (2019) The chemical basis of ferroptosis. Nat Chem Biol 15(12):1137–1147
Kagan VE et al (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13(1):81–90
Lee JY et al (2020) Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci U S A 117(51):32433–32442
Liang D et al (2023) Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186(13):2748-2764.e22
Dixon SJ, Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10(1):9–17
Doll S, Conrad M (2017) Iron and ferroptosis: A still ill-defined liaison. IUBMB Life 69(6):423–434
Shah R, Shchepinov MS, Pratt DA (2018) Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent Sci 4(3):387–396
Aldrovandi M, Fedorova M, Conrad M (2021) Juggling with lipids, a game of Russian roulette. Trends Endocrinol Metab 32(7):463–473
Kagan VE et al (2020) Redox phospholipidomics of enzymatically generated oxygenated phospholipids as specific signals of programmed cell death. Free Radic Biol Med 147:231–241
Gao M et al (2015) Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell 59(2):298–308
Tang D et al (2021) Ferroptosis: molecular mechanisms and health implications. Cell Res 31(2):107–125
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417(1):1–13
Gao M et al (2019) Role of Mitochondria in Ferroptosis. Mol Cell 73(2):354-363.e3
Li C et al (2021) Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy 17(4):948–960
Richardson DR et al (2010) Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci U S A 107(24):10775–10782
Nie G et al (2005) Overexpression of mitochondrial ferritin causes cytosolic iron depletion and changes cellular iron homeostasis. Blood 105(5):2161–2167
Paul BT et al (2017) Mitochondria and Iron: current questions. Expert Rev Hematol 10(1):65–79
Burdo J, Dargusch R, Schubert D (2006) Distribution of the cystine/glutamate antiporter system xc- in the brain, kidney, and duodenum. J Histochem Cytochem 54(5):549–557
Dixon SJ et al (2014) Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3:e02523
Battaglia AM et al (2020) Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells 9(6):1505
Yang WS, Stockwell BR (2016) Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol 26(3):165–176
Friedmann Angeli JP et al (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16(12):1180–1191
Doll S et al (2019) FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575(7784):693–698
Kraft VAN et al (2020) GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci 6(1):41–53
Soula M et al (2020) Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol 16(12):1351–1360
Mao C et al (2021) DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593(7860):586–590
Wang F, Min J (2021) DHODH tangoing with GPX4 on the ferroptotic stage. Signal Transduct Target Ther 6(1):244
Mishima E et al (2023) DHODH inhibitors sensitize to ferroptosis by FSP1 inhibition. Nature 619(7968):E9–E18
Mishima E et al (2022) A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608(7924):778–783
Yang X et al (2023) Regulation of VKORC1L1 is critical for p53-mediated tumor suppression through vitamin K metabolism. Cell Metab 35(8):1474-1490.e8
Kolbrink B et al (2022) Vitamin K1 inhibits ferroptosis and counteracts a detrimental effect of phenprocoumon in experimental acute kidney injury. Cell Mol Life Sci 79(7):387
Jin DY et al (2023) A genome-wide CRISPR-Cas9 knockout screen identifies FSP1 as the warfarin-resistant vitamin K reductase. Nat Commun 14(1):828
Wu M et al (2002) AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J Biol Chem 277(28):25617–25623
Ohiro Y et al (2002) A novel p53-inducible apoptogenic gene, PRG3, encodes a homologue of the apoptosis-inducing factor (AIF). FEBS Lett 524(1–3):163–171
Eisenhaber F et al (2003) Prediction of lipid posttranslational modifications and localization signals from protein sequences: big-Pi, NMT and PTS1. Nucleic Acids Res 31(13):3631–3634
Borgese N et al (1996) A role for N-myristoylation in protein targeting: NADH-cytochrome b5 reductase requires myristic acid for association with outer mitochondrial but not ER membranes. J Cell Biol 135(6):1501–1513
Maurer-Stroh S et al (2004) MYRbase: analysis of genome-wide glycine myristoylation enlarges the functional spectrum of eukaryotic myristoylated proteins. Genome Biol 5(3):R21
Lv Y et al (2023) Structural insights into FSP1 catalysis and ferroptosis inhibition. Nat Commun 14(1):5933
Bai Y et al (2019) Lipid storage and lipophagy regulates ferroptosis. Biochem Biophys Res Commun 508(4):997–1003
Zhu S et al (2017) HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res 77(8):2064–2077
Dai E et al (2020) AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem Biophys Res Commun 523(4):966–971
Nguyen TB et al (2017) DGAT1-Dependent Lipid Droplet Biogenesis Protects Mitochondrial Function during Starvation-Induced Autophagy. Dev Cell 42(1):9-21.e5
Arroyo A et al (1998) Ubiquinol regeneration by plasma membrane ubiquinone reductase. Protoplasma 205(1):107–113
Crane FL (2007) Discovery of ubiquinone (coenzyme Q) and an overview of function. Mitochondrion 7(Suppl):S2-7
Bentinger M, Brismar K, Dallner G (2007) The antioxidant role of coenzyme Q. Mitochondrion 7(Suppl):S41-50
Hadian K (2020) Ferroptosis Suppressor Protein 1 (FSP1) and Coenzyme Q(10) Cooperatively Suppress Ferroptosis. Biochemistry 59(5):637–638
Koppula P et al (2022) A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat Commun 13(1):2206
Kim JW et al (2023) FSP1 confers ferroptosis resistance in KEAP1 mutant non-small cell lung carcinoma in NRF2-dependent and -independent manner. Cell Death Dis 14(8):567
Mukai K, Itoh S, Morimoto H (1992) Stopped-flow kinetic study of vitamin E regeneration reaction with biological hydroquinones (reduced forms of ubiquinone, vitamin K, and tocopherolquinone) in solution. J Biol Chem 267(31):22277–22281
Pedrera L et al (2021) Ferroptotic pores induce Ca(2+) fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ 28(5):1644–1657
Espiritu RA, Pedrera L, Ros U (2019) Tuning the way to die: implications of membrane perturbations in necroptosis. In: IgliÊ A, Garcia-Sáez A, Rappolt M (eds) Advances in biomembranes and lipid self- assembly. Cambridge, Massachusetts: Academic Press.
Jimenez AJ et al (2014) ESCRT Machinery Is Required for Plasma Membrane Repair. Science 343(6174):1247136
Gong YN et al (2017) ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell 169(2):286-300.e16
Gong YN et al (2017) Biological events and molecular signaling following MLKL activation during necroptosis. Cell Cycle 16(19):1748–1760
Rühl S et al (2018) ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362(6417):956–960
Sonder SL et al (2019) Annexin A7 is required for ESCRT III-mediated plasma membrane repair. Sci Rep 9(1):6726
Morita E et al (2007) Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J 26(19):4215–4227
Scheffer LL et al (2014) Mechanism of Ca(2)(+)-triggered ESCRT assembly and regulation of cell membrane repair. Nat Commun 5:5646
Dai E et al (2020) ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem Biophys Res Commun 522(2):415–421
Yang Z et al (2023) HIF-1alpha drives resistance to ferroptosis in solid tumors by promoting lactate production and activating SLC1A1. Cell Rep 42(8):112945
Pontel LB et al (2022) Acute lymphoblastic leukemia necessitates GSH-dependent ferroptosis defenses to overcome FSP1-epigenetic silencing. Redox Biol 55:102408
Liu Y et al (2022) UHRF1-mediated ferroptosis promotes pulmonary fibrosis via epigenetic repression of GPX4 and FSP1 genes. Cell Death Dis 13(12):1070
Muller F et al (2023) Elevated FSP1 protects KRAS-mutated cells from ferroptosis during tumor initiation. Cell Death Differ 30(2):442–456
Donati B, Lorenzini E, Ciarrocchi A (2018) BRD4 and Cancer: going beyond transcriptional regulation. Mol Cancer 17(1):164
Schmitt A et al (2023) BRD4 inhibition sensitizes diffuse large B-cell lymphoma cells to ferroptosis. Blood 142(13):1143–1155
Ebrahimi SO, Reiisi S (2019) Downregulation of miR-4443 and miR-5195-3p in ovarian cancer tissue contributes to metastasis and tumorigenesis. Arch Gynecol Obstet 299(5):1453–1458
Song Z et al (2021) Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci 276:119399
Li M et al (2020) Long Noncoding RNA LINC00460 Promotes Cell Progression by Sponging miR-4443 in Head and Neck Squamous Cell Carcinoma. Cell Transplant 29:963689720927405
Gao Y et al (2019) lncRNA MNX1-AS1 Promotes Glioblastoma Progression Through Inhibition of miR-4443. Oncol Res 27(3):341–347
Gridelli C et al (2018) Cisplatin-Based First-Line Treatment of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: Joint Analysis of MILES-3 and MILES-4 Phase III Trials. J Clin Oncol 36(25):2585–2592
Guo J et al (2018) Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res Treat 50(2):445–460
Griesinger F et al (2019) Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: A meta-analysis. Lung Cancer 135:196–204
Zappa C, Mousa SA (2016) Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 5(3):288–300
MacDonagh L et al (2018) BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett 428:117–126
Montgomery DC et al (2016) Global Profiling of Acetyltransferase Feedback Regulation. J Am Chem Soc 138(20):6388–6391
Sharma S et al (2015) Yeast Kre33 and human NAT10 are conserved 18S rRNA cytosine acetyltransferases that modify tRNAs assisted by the adaptor Tan1/THUMPD1. Nucleic Acids Res 43(4):2242–2258
Tsai K et al (2020) Acetylation of cytidine residues boosts HIV-1 gene expression by increasing viral RNA stability. Cell Host Microbe 28(2):306–312.e6
Zheng X et al (2022) N-acetyltransferase 10 promotes colon cancer progression by inhibiting ferroptosis through N4-acetylation and stabilization of ferroptosis suppressor protein 1 (FSP1) mRNA. Cancer Commun (Lond) 42(12):1347–1366
Dalhat MH et al (2021) Remodelin, a N-acetyltransferase 10 (NAT10) inhibitor, alters mitochondrial lipid metabolism in cancer cells. J Cell Biochem 122(12):1936–1945
Sas-Chen A et al (2020) Dynamic RNA acetylation revealed by quantitative cross-evolutionary mapping. Nature 583(7817):638–643
Balmus G et al (2018) Targeting of NAT10 enhances healthspan in a mouse model of human accelerated aging syndrome. Nat Commun 9(1):1700
Bazhabayi M et al (2021) CircGFRA1 facilitates the malignant progression of HER-2-positive breast cancer via acting as a sponge of miR-1228 and enhancing AIFM2 expression. J Cell Mol Med 25(21):10248–10256
Zhang Y et al (2022) The microRNA-3622 family at the 8p21 locus exerts oncogenic effects by regulating the p53-downstream gene network in prostate cancer progression. Oncogene 41(23):3186–3196
Wang Y (2017) The inhibition of microRNA-15a suppresses hepatitis B virus-associated liver cancer cell growth through the Smad/TGF-beta pathway. Oncol Rep 37(6):3520–3526
Liu MR et al (2023) Sorafenib induces ferroptosis by promoting TRIM54-mediated FSP1 ubiquitination and degradation in hepatocellular carcinoma. Hepatol Commun 7(10):e0246
Yuan J et al (2022) HDLBP-stabilized lncFAL inhibits ferroptosis vulnerability by diminishing Trim69-dependent FSP1 degradation in hepatocellular carcinoma. Redox Biol 58:102546
Gong J et al (2023) TRIM21-Promoted FSP1 Plasma Membrane Translocation Confers Ferroptosis Resistance in Human Cancers. Adv Sci (Weinh) 10:e2302318
Nakamura T et al (2023) Phase separation of FSP1 promotes ferroptosis. Nature 619(7969):371–377
Zhang Q et al (2023) ACSL1-induced ferroptosis and platinum resistance in ovarian cancer by increasing FSP1 N-myristylation and stability. Cell Death Discov 9(1):83
Tao P et al (2021) CYP2J2-produced epoxyeicosatrienoic acids contribute to the ferroptosis resistance of pancreatic ductal adenocarcinoma in a PPARgamma-dependent manner. Zhong Nan Da Xue Xue Bao Yi Xue Ban 46(9):932–941
Efimova I et al (2020) Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer 8(2)
Ma J et al (2023) Composite Hydrogel for Spatiotemporal Lipid Intervention of Tumor Milieu. Adv Mater 35(14):e2211579
Luo X et al (2021) Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ 28(6):1971–1989
Kim KS et al (2022) Enhanced natural killer cell anti-tumor activity with nanoparticles mediated ferroptosis and potential therapeutic application in prostate cancer. J Nanobiotechnol 20(1):428
Zhang H et al (2022) Dihydroartemisinin inhibits the growth of pancreatic cells by inducing ferroptosis and activating antitumor immunity. Eur J Pharmacol 926:175028
Wiernicki B et al (2022) Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor immunity. Nat Commun 13(1):3676
Mbah NE, Lyssiotis CA (2022) Metabolic regulation of ferroptosis in the tumor microenvironment. J Biol Chem 298(3):101617
Hayes JD, Dinkova-Kostova AT, Tew KD (2020) Oxidative Stress in Cancer. Cancer Cell 38(2):167–197
Spranger S et al (2017) Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 31(5):711-723.e4
Conche C et al (2023) Combining ferroptosis induction with MDSC blockade renders primary tumours and metastases in liver sensitive to immune checkpoint blockade. Gut 72(9):1774–1782
Zang J et al (2023) Overexpression of ferroptosis-related genes FSP1 and CISD1 is related to prognosis and tumor immune infiltration in gastric cancer. Clin Transl Oncol 25(8):2532–2544
Cheu JW et al (2023) Ferroptosis Suppressor Protein 1 Inhibition Promotes Tumor Ferroptosis and Anti-tumor Immune Responses in Liver Cancer. Cell Mol Gastroenterol Hepatol 16(1):133–159
Drijvers JM et al (2021) Pharmacologic Screening Identifies Metabolic Vulnerabilities of CD8(+) T Cells. Cancer Immunol Res 9(2):184–199
Xavier da Silva TN et al (2023) Molecular characterization of AIFM2/FSP1 inhibition by iFSP1-like molecules. Cell Death Dis 14(4):281
Cheu JW et al (2023) Ferroptosis suppressor protein 1 inhibition promotes tumor ferroptosis and anti-tumor immune responses in liver cancer. Cell Mol Gastroenterol Hepatol 16(1):133–159
Ozga AJ, Chow MT, Luster AD (2021) Chemokines and the immune response to cancer. Immunity 54(5):859–874
House IG et al (2020) Macrophage-Derived CXCL9 and CXCL10 Are Required for Antitumor Immune Responses Following Immune Checkpoint Blockade. Clin Cancer Res 26(2):487–504
Truman LA et al (2008) CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 112(13):5026–5036
Yamasaki M et al (2010) p53 genotype predicts response to chemotherapy in patients with squamous cell carcinoma of the esophagus. Ann Surg Oncol 17(2):634–642
Miyauchi W et al (2022) Simultaneous regulation of ferroptosis suppressor protein 1 and glutathione peroxidase 4 as a new therapeutic strategy of ferroptosis for esophageal squamous cell carcinoma. Esophagus-Tokyo 20(3):492–501
Qiu C et al (2022) Novel Therapeutic Savior for Osteosarcoma: The Endorsement of Ferroptosis. Front Oncol 12:746030
Nakamura T et al (2023) Integrated chemical and genetic screens unveil FSP1 mechanisms of ferroptosis regulation. Nat Struct Mol Biol 30(11):1806–1815
Hendricks JM et al (2023) Identification of structurally diverse FSP1 inhibitors that sensitize cancer cells to ferroptosis. Cell Chem Biol 30(9):1090-1103.e7
Yoshioka H et al (2022) Identification of a Small Molecule That Enhances Ferroptosis via Inhibition of Ferroptosis Suppressor Protein 1 (FSP1). ACS Chem Biol 17(2):483–491
Zhao X et al (2023) A chiral fluorescent Ir(iii) complex that targets the GPX4 and ErbB pathways to induce cellular ferroptosis. Chem Sci 14(5):1114–1122
Cao H et al (2023) QiLing Decoction promotes ferroptosis of castration-resistant prostate cancer cells by inhibiting FSP1 in vitro and in vivo. J Cancer 14(12):2236–2245
Chen X et al (2022) Ferroptosis Induction Improves the Sensitivity of Docetaxel in Prostate Cancer. Oxid Med Cell Longev 2022:1–16
Zhou J et al (2023) Curcumin Induces Ferroptosis in A549 CD133(+) Cells through the GSH-GPX4 and FSP1-CoQ10-NAPH Pathways. Discov Med 35(176):251–263
Wan MohdTajuddin WNB et al (2019) Mechanistic Understanding of Curcumin’s Therapeutic Effects in Lung Cancer. Nutrients 11(12):2989
Miyazaki K et al (2023) Curcumin and Andrographis Exhibit Anti-Tumor Effects in Colorectal Cancer via Activation of Ferroptosis and Dual Suppression of Glutathione Peroxidase-4 and Ferroptosis Suppressor Protein-1. Pharmaceuticals (Basel) 16(3):383
Sessler DI, Riedel B (2019) Anesthesia and Cancer Recurrence: Context for Divergent Study Outcomes. Anesthesiology 130(1):3–5
Zhao MY et al (2022) Propofol Augments Paclitaxel-Induced Cervical Cancer Cell Ferroptosis In Vitro. Front Pharmacol 13:816432
Utsumi T et al (2018) Identification and characterization of protein N-myristoylation occurring on four human mitochondrial proteins, SAMM50, TOMM40, MIC19, and MIC25. PLoS ONE 13(11):e0206355
Fedoryshchak RO et al (2023) Discovery of lipid-mediated protein-protein interactions in living cells using metabolic labeling with photoactivatable clickable probes. Chem Sci 14(9):2419–2430
Li K et al (2022) Multienzyme-like Reactivity Cooperatively Impairs Glutathione Peroxidase 4 and Ferroptosis Suppressor Protein 1 Pathways in Triple-Negative Breast Cancer for Sensitized Ferroptosis Therapy. ACS nano 16(2):2381–2398
Zhu P, Chen Y, Shi J (2018) Nanoenzyme-Augmented Cancer Sonodynamic Therapy by Catalytic Tumor Oxygenation. ACS Nano 12(4):3780–3795
Zeng Z et al (2021) Activatable Polymer Nanoenzymes for Photodynamic Immunometabolic Cancer Therapy. Adv Mater 33(4):e2007247
Yu Z et al (2020) Nanoenzymes in disease diagnosis and therapy. Chem Commun (Camb) 56(99):15513–15524
Yang J et al (2022) Metabolic Intervention Nanoparticles for Triple-Negative Breast Cancer Therapy via Overcoming FSP1-Mediated Ferroptosis Resistance. Adv Healthc Mater 11(13):e2102799
Stockwell BR, Jiang X (2020) The Chemistry and Biology of Ferroptosis. Cell Chem Biol 27(4):365–375
Beckwitt CH et al (2018) Statins attenuate outgrowth of breast cancer metastases. Br J Cancer 119(9):1094–1105
Fromigue O, Hamidouche Z, Marie PJ (2008) Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces osteosarcoma cell invasion. J Biol Chem 283(45):30549–30556
Tan M et al (2019) Silk Fibroin-Coated Nanoagents for Acidic Lysosome Targeting by a Functional Preservation Strategy in Cancer Chemotherapy. Theranostics 9(4):961–973
Delaney G et al (2005) The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104(6):1129–1137
Azzam EI, Jay-Gerin JP, Pain D (2012) Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 327(1–2):48–60
Reisz JA et al (2014) Effects of ionizing radiation on biological molecules–mechanisms of damage and emerging methods of detection. Antioxid Redox Signal 21(2):260–292
Baidoo KE, Yong K, Brechbiel MW (2013) Molecular pathways: targeted alpha-particle radiation therapy. Clin Cancer Res 19(3):530–537
Adjemian S et al (2020) Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death. Cell Death Dis 11(11):1003
Lei G et al (2021) Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell 12(11):836–857
Venkatesh D et al (2020) MDM2 and MDMX promote ferroptosis by PPARalpha-mediated lipid remodeling. Genes Dev 34(7–8):526–543
Zou Y, Schreiber SL (2020) Progress in Understanding Ferroptosis and Challenges in Its Targeting for Therapeutic Benefit. Cell Chem Biol 27(4):463–471
Tang W et al (2020) The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther 5(1):87
Zhao L et al (2022) Ferroptosis in cancer and cancer immunotherapy. Cancer Commun (Lond) 42(2):88–116
Zhou Y et al (2023) A green light-enhanced cytosolic protein delivery platform based on BODIPY-protein interactions. Nano Res 16(1):1042–1051
Zhou Y et al (2023) Photo-Enhanced Synergistic Induction of Ferroptosis for Anti-Cancer Immunotherapy. Adv Healthc Mater 12:e2300994
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82303270), the Excellent Young Medical Talents Training Fund of the First Affiliated Hospital of Harbin Medical University (Grant No. 2021Y06), Heilongjiang Postdoctoral Scientific Research Developmental Fund (Grant No. LBH-Q18089), the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (Grant No. UNPYSCT-2018070), Chen Xiao-Ping Foundation For The Development Of Science And Technology Of Hubei Province (Grant No. CXPJJH121001-2021029).
Author information
Authors and Affiliations
Contributions
RG and JGW conceived and drafted the manuscript, drew the figures, and summarized the tables. JJH, TW, LFG, WLL, and JLG discussed the concepts of the manuscript. DSL, QHM and HYP provided valuable suggestion. HYP approved the submission of the manuscript RG and JGW contributed equally to this work.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agreed with the content of the manuscript.
Competing interests
The authors have declared that no competing interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, R., Wang, J., Huang, J. et al. FSP1-mediated ferroptosis in cancer: from mechanisms to therapeutic applications. Apoptosis (2024). https://doi.org/10.1007/s10495-024-01966-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s10495-024-01966-1