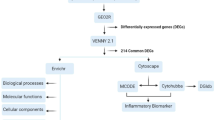
Abstract
Atherosclerosis (AS) is a pathological process associated with various cardiovascular diseases. Upon different stimuli, neutrophils release reticular complexes known as neutrophil extracellular traps (NETs). Numerous researches have indicated a strong correlation between NETs and AS. However, its role in cardiovascular disease requires further investigation. By utilizing a machine learning algorithm, we examined the genes associated with NETs that were expressed differently in individuals with AS compared to normal controls. As a result, we identified four distinct genes. A nomogram model was built to forecast the incidence of AS. Additionally, we conducted analysis on immune infiltration, functional enrichment and consensus clustering in AS samples. The findings indicated that individuals with AS could be categorized into two groups, exhibiting notable variations in immune infiltration traits among the groups. Furthermore, to measure the NETs model, the principal component analysis algorithm was developed and cluster B outperformed cluster A in terms of NETs. Additionally, there were variations in the expression of multiple chemokines between the two subtypes. By studying AS NETs, we acquired fresh knowledge about the molecular patterns and immune mechanisms implicated, which could open up new possibilities for AS immunotherapy.








Similar content being viewed by others
Data availability
Both the GEO database (https://www.ncbi.nlm.nih.gov/geo/) and the published article contain the data that substantiate the findings of this research. All analysis methods and software packages were indicated in the section titled “Materials and methods”.
References
Herrington W, Lacey B, Sherliker P et al (2016) Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res 118:535–546
Gallino A, Aboyans V, Diehm C et al (2014) Non-coronary atherosclerosis. Eur Heart J 35:1112–1119
Badimon L, Vilahur G (2014) Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med 276:618–632
Arts EEA, Popa C, Den Broeder AA et al (2015) Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis 74:668–674
Bathon JM, Centola M, Liu X et al (2023) Identification of novel biomarkers for the prediction of subclinical coronary artery atherosclerosis in patients with rheumatoid arthritis: an exploratory analysis. Arthritis Res Ther 25:213
Filep JG (2022) Targeting neutrophils for promoting the resolution of inflammation. Front Immunol 13:866747
Yang YW, Deng NH, Tian KJ et al (2022) Development of hydrogen sulfide donors for anti-atherosclerosis therapeutics research: challenges and future priorities. Front Cardiovasc Med 9:909178
Roy P, Orecchioni M, Ley K (2022) How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat Rev Immunol 22:251–265
Brinkmann V, Reichard U, Goosmann C et al (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535
Megens RTA, Vijayan S, Lievens D et al (2012) Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb Haemost 107:597–598
Knight JS, Luo W, O’Dell AA et al (2014) Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res 114:947–956
Carmona-Rivera C, Zhao W, Yalavarthi S et al (2015) Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 74:1417–1424
Warnatsch A, Ioannou M, Wang Q et al (2015) Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349:316–320
Zhang Y, Jian W, He L et al (2020) Externalized histone H4: a novel target that orchestrates chronic inflammation by inducing lytic cell death. Acta Biochim Biophys Sin 52:336–338
Döring Y, Manthey HD, Drechsler M et al (2012) Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 125:1673–1683
Zhang R, Brennan ML, Fu X et al (2001) Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 286(17):2136–2142
Mangold A, Alias S, Scherz T et al (2015) Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res 116:1182–1192
Helseth R, Shetelig C, Andersen GØ et al (2019) Neutrophil extracellular trap components associate with infarct size, ventricular function, and clinical outcome in STEMI. Mediat Inflamm. https://doi.org/10.1155/2019/7816491
Langseth MS, Opstad TB, Bratseth V et al (2018) Markers of neutrophil extracellular traps are associated with adverse clinical outcome in stable coronary artery disease. Eur J Prev Cardiol 25:762–769
Kithcart AP, Libby P (2018) Casting NETs to predict cardiovascular outcomes. Eur J Prev Cardiol 25:759–761
Steenman M, Espitia O, Maurel B et al (2018) Identification of genomic differences among peripheral arterial beds in atherosclerotic and healthy arteries. Sci Rep 8:3940
Ayari H, Bricca G (2013) Identification of two genes potentially associated in iron-heme homeostasis in human carotid plaque using microarray analysis. J Biosci 38:311–315
Jin H, Goossens P, Juhasz P et al (2021) Integrative multiomics analysis of human atherosclerosis reveals a serum response factor-driven network associated with intraplaque hemorrhage. Clin Transl Med 11:e458
Wu J, Zhang F, Zheng X et al (2022) Identification of renal ischemia reperfusion injury subtypes and predictive strategies for delayed graft function and graft survival based on neutrophil extracellular trap-related genes. Front Immunol 13:1047367
Ritchie ME, Phipson B, Wu DI et al (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47–e47
Engebretsen S, Bohlin J (2019) Statistical predictions with glmnet. Clin Epigenet 11:1–3
Lin X, Li C, Zhang Y et al (2017) Selecting feature subsets based on SVM-RFE and the overlapping ratio with applications in bioinformatics. Molecules 23:52
Alderden J, Pepper GA, Wilson A et al (2018) Predicting pressure injury in critical care patients: a machine-learning model. Am J Crit Care 27:461–468
Iasonos A, Schrag D, Raj GV et al (2008) How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26:1364–1370
Robin X, Turck N, Hainard A et al (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform 12:1–8
Zhang Y, Liu N, Wang S (2018) A differential privacy protecting K-means clustering algorithm based on contour coefficients. PLoS ONE 13:e0206832
Hänzelmann S, Castelo R, Guinney J (2013) GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform 14:1–15
Charoentong P, Finotello F, Angelova M et al (2017) Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep 18:248–262
Zhang B, Wu Q, Li B et al (2020) m6A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer 19:1–21
Roth GA, Johnson C, Abajobir A et al (2017) Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70:1–25
Döring Y, Soehnlein O, Weber C (2017) Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res 120:736–743
Tian Z, Li X, Jiang D (2023) Analysis of immunogenic cell death in atherosclerosis based on scRNA-seq and bulk RNA-seq data. Int Immunopharmacol 119:110130
Tang C, Deng L, Luo Q et al (2023) Identification of oxidative stress-related genes and potential mechanisms in atherosclerosis. Front Genet 13:998954
Urban CF, Ermert D, Schmid M et al (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5:e1000639
Briggs RC, Atkinson JB, Miranda RN (2005) Variable expression of human myeloid specific nuclear antigen MNDA in monocyte lineage cells in atherosclerosis. J Cell Biochem 95:293–301
Lin JD, Nishi H, Poles J et al (2019) Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. https://doi.org/10.1172/jci.insight.124574
Boshuizen MCS, de Winther MPJ (2015) Interferons as essential modulators of atherosclerosis. Arterioscler Thromb Vasc Biol 35:1579–1588
Manthey HD, Thomas AC, Shiels IA et al (2011) Complement C5a inhibition reduces atherosclerosis in ApoE–/–mice. FASEB J 25:2447–2455
An G, Li B, Liu X et al (2016) Overexpression of complement component C5a accelerates the development of atherosclerosis in ApoE-knockout mice. Oncotarget 7:56060
Libby P (2017) Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol 70:2278–2289
Keshari RS, Jyoti A, Dubey M et al (2012) Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS ONE 7:e48111
Fu L, Liu Z, Liu Y (2023) Fibrinogen-like protein 2 in inflammatory diseases: a future therapeutic target. Int Immunopharmacol 116:109799
Herrero-Fernandez B, Gomez-Bris R, Somovilla-Crespo B et al (2019) Immunobiology of atherosclerosis: a complex net of interactions. Int J Mol Sci 20:5293
Yang Y, Yi X, Cai Y et al (2022) Immune-associated gene signatures and subtypes to predict the progression of atherosclerotic plaques based on machine learning. Front Pharmacol 13:865624
Yan Y, Thakur M, van der Vorst EPC et al (2021) Targeting the chemokine network in atherosclerosis. Atherosclerosis 330:95–106
Georgakis MK, Malik R, Björkbacka H et al (2019) Circulating monocyte chemoattractant protein-1 and risk of stroke: meta-analysis of population-based studies involving 17 180 individuals. Circ Res 125:773–782
Combadière C, Potteaux S, Rodero M et al (2008) Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117:1649–1657
Boring L, Gosling J, Cleary M et al (1988) Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394(6696):894–897
Gutwein P, Abdel-Bakky MS, Schramme A et al (2009) CXCL16 is expressed in podocytes and acts as a scavenger receptor for oxidized low-density lipoprotein. Am J Pathol 174:2061–2072
dos Santos SM, Blankenbach K, Scholich K et al (2015) Platelets from flowing blood attach to the inflammatory chemokine CXCL16 expressed in the endothelium of the human vessel wall. Thromb Haemost 114:297–312
Linke B, Dos Santos SM, Picard-Willems B et al (2019) CXCL16/CXCR6-mediated adhesion of human peripheral blood mononuclear cells to inflamed endothelium. Cytokine 122:154081
van den Borne P, Quax PHA, Hoefer IE et al (2011) The multifaceted functions of CXCL10 in cardiovascular disease. BioMed Res Int. https://doi.org/10.1155/2014/893106
Segers D, Lipton JA, Leenen PJM et al (2011) Atherosclerotic plaque stability is affected by the chemokine CXCL10 in both mice and humans. Int J Inflamm. https://doi.org/10.4061/2011/936109
Funding
As part of the project, we received funds from the Hebei Province Education Department (Project No. QN2016145), as well as the University-level Science Funds in CDMC (202118 and 2023108), and the Chengde Medical University’s Fundamental Research Funds (KY202220). Thanks for these help, we were also honoured to be rated as a national college student scientific research project (Project No. 2023004).
Author information
Authors and Affiliations
Contributions
The study’s planning and manuscript writing were done by LS and BZ. The data was collected and examined by RL, YD and YC.The final paper was examined and approved by YX and XH. QX oversaw all aspects of the project, including data interpretation and article review. After evaluating the findings, all authors gave their approval to the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
Throughout the study, the authors assert that there were no conflicts of interest, either in terms of relationships or financial ties, that could be perceived as potential.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, L., Zhang, B., Li, R. et al. Significance of neutrophil extracellular traps-related gene in the diagnosis and classification of atherosclerosis. Apoptosis 29, 605–619 (2024). https://doi.org/10.1007/s10495-023-01923-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01923-4