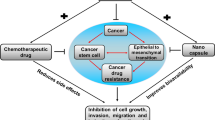
Abstract
Cancer has presented to be the most challenging disease, contributing to one in six mortalities worldwide. The current treatment regimen involves multiple rounds of chemotherapy administration, alone or in combination. The treatment has adverse effects including cardiomyopathy, hepatotoxicity, and nephrotoxicity. In addition, the development of resistance to chemo has been attributed to cancer relapse and low patient overall survivability. Multiple drug resistance development may be through numerous factors such as up-regulation of drug transporters, drug inactivation, alteration of drug targets and drug degradation. Doxorubicin is a widely used first line chemotherapeutic drug for a myriad of cancers. It has multiple intracellular targets, DNA intercalation, adduct formation, topoisomerase inhibition, iron chelation, reactive oxygen species generation and promotes immune mediated clearance of the tumor. Agents that can sensitize the resistant cancer cells to the chemotherapeutic drug are currently the focus to improve the clinical efficiency of cancer therapy. This review summarizes the recent 10-year research on the use of natural phytochemicals, inhibitors of apoptosis and autophagy, miRNAs, siRNAs and nanoformulations being investigated for doxorubicin chemosensitization.





Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Wilson BE, Jacob S, Yap ML, Ferlay J, Bray F, Barton MB (2019) Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: a population-based study. Lancet Oncol 20(6):769–780. https://doi.org/10.1016/S1470-2045(19)30163-9
Mathur P, Sathishkumar K, Chaturvedi M, Das P, Sudarshan KL, Santhappan S, Nallasamy V, John A, Narasimhan S, Roselind FS, Group I-N-NI (2020) Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob Oncol 6:1063–1075. https://doi.org/10.1200/GO.20.00122
Guo W, Tan HY, Chen F, Wang N, Feng Y (2020) Targeting cancer metabolism to resensitize chemotherapy: potential development of cancer chemosensitizers from traditional Chinese medicines. Cancers (Basel). https://doi.org/10.3390/cancers12020404
Cortes-Funes H, Coronado C (2007) Role of anthracyclines in the era of targeted therapy. Cardiovasc Toxicol 7(2):56–60. https://doi.org/10.1007/s12012-007-0015-3
Yang F, Teves SS, Kemp CJ, Henikoff S (2014) Doxorubicin, DNA torsion, and chromatin dynamics. Biochim Biophys Acta 1845(1):84–89. https://doi.org/10.1016/j.bbcan.2013.12.002
Gammella E, Maccarinelli F, Buratti P, Recalcati S, Cairo G (2014) The role of iron in anthracycline cardiotoxicity. Front Pharmacol 5:25. https://doi.org/10.3389/fphar.2014.00025
Piska K, Koczurkiewicz P, Bucki A, Wojcik-Pszczola K, Kolaczkowski M, Pekala E (2017) Metabolic carbonyl reduction of anthracyclines—role in cardiotoxicity and cancer resistance. Reducing enzymes as putative targets for novel cardioprotective and chemosensitizing agents. Invest New Drugs 35(3):375–385. https://doi.org/10.1007/s10637-017-0443-2
Edwardson DW, Narendrula R, Chewchuk S, Mispel-Beyer K, Mapletoft JP, Parissenti AM (2015) Role of drug metabolism in the cytotoxicity and clinical efficacy of anthracyclines. Curr Drug Metab 16(6):412–426. https://doi.org/10.2174/1389200216888150915112039
Allen TM, Cullis PR (2013) Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 65(1):36–48. https://doi.org/10.1016/j.addr.2012.09.037
Chang A (2011) Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer 71(1):3–10. https://doi.org/10.1016/j.lungcan.2010.08.022
Seton-Rogers S (2016) Chemotherapy: preventing competitive release. Nat Rev Cancer 16(4):199. https://doi.org/10.1038/nrc.2016.28
Pan ST, Li ZL, He ZX, Qiu JX, Zhou SF (2016) Molecular mechanisms for tumour resistance to chemotherapy. Clin Exp Pharmacol Physiol 43(8):723–737. https://doi.org/10.1111/1440-1681.12581
Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB (2011) Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genom 21(7):440–446. https://doi.org/10.1097/FPC.0b013e32833ffb56
Arcamone F (1987) Clinically useful doxorubicin analogues. Cancer Treat Rev 14(3–4):159–161. https://doi.org/10.1016/0305-7372(87)90002-8
Korcok M (1985) American symposium looks at new antineoplastic drug. Can Med Assoc J 133(1):66–67
Qi HP, Gao XC, Zhang LQ, Wei SQ, Bi S, Yang ZC, Cui H (2013) In vitro evaluation of enhancing effect of borneol on transcorneal permeation of compounds with different hydrophilicities and molecular sizes. Eur J Pharmacol 705(1–3):20–25. https://doi.org/10.1016/j.ejphar.2013.02.031
Cao WQ, Li Y, Hou YJ, Yang MX, Fu XQ, Zhao BS, Jiang HM, Fu XY (2019) Enhanced anticancer efficiency of doxorubicin against human glioma by natural borneol through triggering ROS-mediated signal. Biomed Pharmacother 118:109261. https://doi.org/10.1016/j.biopha.2019.109261
Fan X, Chai L, Zhang H, Wang Y, Zhang B, Gao X (2015) Borneol depresses P-glycoprotein function by a NF-kappaB signaling mediated mechanism in a blood brain barrier in vitro model. Int J Mol Sci 16(11):27576–27588. https://doi.org/10.3390/ijms161126051
Meng L, Chu X, Xing H, Liu X, Xin X, Chen L, Jin M, Guan Y, Huang W, Gao Z (2019) Improving glioblastoma therapeutic outcomes via doxorubicin-loaded nanomicelles modified with borneol. Int J Pharm 567:118485. https://doi.org/10.1016/j.ijpharm.2019.118485
Lai H, Liu C, Hou L, Lin W, Chen T, Hong A (2020) TRPM8-regulated calcium mobilization plays a critical role in synergistic chemosensitization of Borneol on Doxorubicin. Theranostics 10(22):10154–10170. https://doi.org/10.7150/thno.45861
Yang R, Chen Z, Xie F, Xie M, Liu N, Su Z, Gu J, Zhao R (2021) (+/-)-Borneol reverses mitoxantrone resistance against P-glycoprotein. J Chem Inf Model 61(1):252–262. https://doi.org/10.1021/acs.jcim.0c00892
Zou L, Wang D, Hu Y, Fu C, Li W, Dai L, Yang L, Zhang J (2017) Drug resistance reversal in ovarian cancer cells of paclitaxel and borneol combination therapy mediated by PEG-PAMAM nanoparticles. Oncotarget 8(36):60453–60468. https://doi.org/10.18632/oncotarget.19728
Wang S, Tan N, Ma C, Wang J, Jia P, Liu J, Yang Y, Xie Z, Zhao K, Zheng X (2018) Inhibitory effects of benzaldehyde, vanillin, muscone and borneol on P-glycoprotein in Caco-2 cells and everted gut Sac. Pharmacology 101(5–6):269–277. https://doi.org/10.1159/000487144
Liu WJ, Yin YB, Sun JY, Feng S, Ma JK, Fu XY, Hou YJ, Yang MF, Sun BL, Fan CD (2018) Natural borneol is a novel chemosensitizer that enhances temozolomide-induced anticancer efficiency against human glioma by triggering mitochondrial dysfunction and reactive oxide species-mediated oxidative damage. Onco Targets Ther 11:5429–5439. https://doi.org/10.2147/OTT.S174498
Cao WQ, Zhai XQ, Ma JW, Fu XQ, Zhao BS, Zhang P, Fu XY (2020) Natural borneol sensitizes human glioma cells to cisplatin-induced apoptosis by triggering ROS-mediated oxidative damage and regulation of MAPKs and PI3K/AKT pathway. Pharm Biol 58(1):72–79. https://doi.org/10.1080/13880209.2019.1703756
He Y, Ding J, Lin Y, Li J, Shi Y, Wang J, Zhu Y, Wang K, Hu X (2015) Gambogenic acid alters chemosensitivity of breast cancer cells to Adriamycin. BMC Complement Altern Med 15:181. https://doi.org/10.1186/s12906-015-0710-8
Xu Q, Guo J, Chen W (2019) Gambogenic acid reverses P-glycoprotein mediated multidrug resistance in HepG2/Adr cells and its underlying mechanism. Biochem Biophys Res Commun 508(3):882–888. https://doi.org/10.1016/j.bbrc.2018.12.028
Shen D, Wang Y, Niu H, Liu C (2020) Gambogenic acid exerts anticancer effects in cisplatinresistant nonsmall cell lung cancer cells. Mol Med Rep 21(3):1267–1275. https://doi.org/10.3892/mmr.2020.10909
Xu L, Meng X, Xu N, Fu W, Tan H, Zhang L, Zhou Q, Qian J, Tu S, Li X, Lao Y, Xu H (2018) Gambogenic acid inhibits fibroblast growth factor receptor signaling pathway in erlotinib-resistant non-small-cell lung cancer and suppresses patient-derived xenograft growth. Cell Death Dis 9(3):262. https://doi.org/10.1038/s41419-018-0314-6
Yu XJ, Han QB, Wen ZS, Ma L, Gao J, Zhou GB (2012) Gambogenic acid induces G1 arrest via GSK3beta-dependent cyclin D1 degradation and triggers autophagy in lung cancer cells. Cancer Lett 322(2):185–194. https://doi.org/10.1016/j.canlet.2012.03.004
Jang J, Jeong SJ, Kwon HY, Jung JH, Sohn EJ, Lee HJ, Kim JH, Kim SH, Kim JH, Kim SH (2013) Decursin and doxorubicin are in synergy for the induction of apoptosis via STAT3 and/or mTOR pathways in human multiple myeloma cells. Evid Based Complement Alternat Med 2013:506324. https://doi.org/10.1155/2013/506324
Choi HS, Cho SG, Kim MK, Kim MS, Moon SH, Kim IH, Ko SG (2016) Decursin in Angelica gigas Nakai (AGN) enhances doxorubicin chemosensitivity in NCI/ADR-RES ovarian cancer cells via inhibition of P-glycoprotein expression. Phytother Res 30(12):2020–2026. https://doi.org/10.1002/ptr.5708
Kim HJ, Kim SM, Park KR, Jang HJ, Na YS, Ahn KS, Kim SH, Ahn KS (2011) Decursin chemosensitizes human multiple myeloma cells through inhibition of STAT3 signaling pathway. Cancer Lett 301(1):29–37. https://doi.org/10.1016/j.canlet.2010.11.002
Piska K, Koczurkiewicz P, Wnuk D, Karnas E, Bucki A, Wojcik-Pszczola K, Jamrozik M, Michalik M, Kolaczkowski M, Pekala E (2019) Synergistic anticancer activity of doxorubicin and piperlongumine on DU-145 prostate cancer cells—the involvement of carbonyl reductase 1 inhibition. Chem Biol Interact 300:40–48. https://doi.org/10.1016/j.cbi.2019.01.003
Chen D, Ma Y, Li P, Liu M, Fang Y, Zhang J, Zhang B, Hui Y, Yin Y (2019) Piperlongumine induces apoptosis and synergizes with doxorubicin by inhibiting the JAK2-STAT3 pathway in triple-negative breast cancer. Molecules. https://doi.org/10.3390/molecules24122338
Kang Q, Yan S (2015) Piperlongumine reverses doxorubicin resistance through the PI3K/Akt signaling pathway in K562/A02 human leukemia cells. Exp Ther Med 9(4):1345–1350. https://doi.org/10.3892/etm.2015.2254
Yao Y, Sun Y, Shi M, Xia D, Zhao K, Zeng L, Yao R, Zhang Y, Li Z, Niu M, Xu K (2016) Piperlongumine induces apoptosis and reduces bortezomib resistance by inhibiting STAT3 in multiple myeloma cells. Oncotarget 7(45):73497–73508. https://doi.org/10.18632/oncotarget.11988
Qian J, Xu Z, Zhu P, Meng C, Liu Y, Shan W, He A, Gu Y, Ran F, Zhang Y, Ling Y (2021) A derivative of piperlongumine and ligustrazine as a potential thioredoxin reductase inhibitor in drug-resistant hepatocellular carcinoma. J Nat Prod 84(12):3161–3168. https://doi.org/10.1021/acs.jnatprod.1c00618
Chen W, Lian W, Yuan Y, Li M (2019) The synergistic effects of oxaliplatin and piperlongumine on colorectal cancer are mediated by oxidative stress. Cell Death Dis 10(8):600. https://doi.org/10.1038/s41419-019-1824-6
Zhang C, He LJ, Zhu YB, Fan QZ, Miao DD, Zhang SP, Zhao WY, Liu XP (2019) Piperlongumine inhibits Akt phosphorylation to reverse resistance to cisplatin in human non-small cell lung cancer cells via ROS regulation. Front Pharmacol 10:1178. https://doi.org/10.3389/fphar.2019.01178
Wang F, Mao Y, You Q, Hua D, Cai D (2015) Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int J Immunopathol Pharmacol 28(3):362–373. https://doi.org/10.1177/0394632015598849
Wen C, Fu L, Huang J, Dai Y, Wang B, Xu G, Wu L, Zhou H (2019) Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicinresistant breast cancer cells. Mol Med Rep 19(6):5162–5168. https://doi.org/10.3892/mmr.2019.10180
Zhao X, Chen Q, Liu W, Li Y, Tang H, Liu X, Yang X (2015) Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer. Int J Nanomed 10:257–270. https://doi.org/10.2147/IJN.S73322
Pimentel-Gutierrez HJ, Bobadilla-Morales L, Barba-Barba CC, Ortega-De-La-Torre C, Sanchez-Zubieta FA, Corona-Rivera JR, Gonzalez-Quezada BA, Armendariz-Borunda JS, Silva-Cruz R, Corona-Rivera A (2016) Curcumin potentiates the effect of chemotherapy against acute lymphoblastic leukemia cells via downregulation of NF-kappaB. Oncol Lett 12(5):4117–4124. https://doi.org/10.3892/ol.2016.5217
Fathy Abd-Ellatef GE, Gazzano E, Chirio D, Hamed AR, Belisario DC, Zuddas C, Peira E, Rolando B, Kopecka J, Assem Said Marie M, Sapino S, Ramadan Fahmy S, Gallarate M, Abdel-Hamid AZ, Riganti C (2020) Curcumin-loaded solid lipid nanoparticles bypass P-glycoprotein mediated doxorubicin resistance in triple negative breast cancer cells. Pharmaceutics. https://doi.org/10.3390/pharmaceutics12020096
Zhao MD, Li JQ, Chen FY, Dong W, Wen LJ, Fei WD, Zhang X, Yang PL, Zhang XM, Zheng CH (2019) Co-delivery of curcumin and paclitaxel by “Core-Shell” targeting amphiphilic copolymer to reverse resistance in the treatment of ovarian cancer. Int J Nanomed 14:9453–9467. https://doi.org/10.2147/IJN.S224579
Muhanmode Y, Wen MK, Maitinuri A, Shen G (2021) Curcumin and resveratrol inhibit chemoresistance in cisplatin-resistant epithelial ovarian cancer cells via targeting P13K pathway. Hum Exp Toxicol 40(12_suppl 1):S861–S868. https://doi.org/10.1177/09603271211052985
Sung B, Kunnumakkara AB, Sethi G, Anand P, Guha S, Aggarwal BB (2009) Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol Cancer Ther 8(4):959–970. https://doi.org/10.1158/1535-7163.MCT-08-0905
Howells LM, Sale S, Sriramareddy SN, Irving GR, Jones DJ, Ottley CJ, Pearson DG, Mann CD, Manson MM, Berry DP, Gescher A, Steward WP, Brown K (2011) Curcumin ameliorates oxaliplatin-induced chemoresistance in HCT116 colorectal cancer cells in vitro and in vivo. Int J Cancer 129(2):476–486. https://doi.org/10.1002/ijc.25670
Wei Y, Yang P, Cao S, Zhao L (2018) The combination of curcumin and 5-fluorouracil in cancer therapy. Arch Pharm Res 41(1):1–13. https://doi.org/10.1007/s12272-017-0979-x
Hajra S, Patra AR, Basu A, Saha P, Bhattacharya S (2018) Indole-3-Carbinol (I3C) enhances the sensitivity of murine breast adenocarcinoma cells to doxorubicin (DOX) through inhibition of NF-kappabeta, blocking angiogenesis and regulation of mitochondrial apoptotic pathway. Chem Biol Interact 290:19–36. https://doi.org/10.1016/j.cbi.2018.05.005
Hajra S, Patra AR, Basu A, Bhattacharya S (2018) Prevention of doxorubicin (DOX)-induced genotoxicity and cardiotoxicity: Effect of plant derived small molecule indole-3-carbinol (I3C) on oxidative stress and inflammation. Biomed Pharmacother 101:228–243. https://doi.org/10.1016/j.biopha.2018.02.088
Adwas AA, Elkhoely AA, Kabel AM, Abdel-Rahman MN, Eissa AA (2016) Anti-cancer and cardioprotective effects of indol-3-carbinol in doxorubicin-treated mice. J Infect Chemother 22(1):36–43. https://doi.org/10.1016/j.jiac.2015.10.001
Taylor-Harding B, Agadjanian H, Nassanian H, Kwon S, Guo X, Miller C, Karlan BY, Orsulic S, Walsh CS (2012) Indole-3-carbinol synergistically sensitises ovarian cancer cells to bortezomib treatment. Br J Cancer 106(2):333–343. https://doi.org/10.1038/bjc.2011.546
Paik WH, Kim HR, Park JK, Song BJ, Lee SH, Hwang JH (2013) Chemosensitivity induced by down-regulation of microRNA-21 in gemcitabine-resistant pancreatic cancer cells by indole-3-carbinol. Anticancer Res 33(4):1473–1481
Hassan S, Peluso J, Chalhoub S, Idoux Gillet Y, Benkirane-Jessel N, Rochel N, Fuhrmann G, Ubeaud-Sequier G (2020) Quercetin potentializes the respective cytotoxic activity of gemcitabine or doxorubicin on 3D culture of AsPC-1 or HepG2 cells, through the inhibition of HIF-1alpha and MDR1. PLoS ONE 15(10):e0240676. https://doi.org/10.1371/journal.pone.0240676
Lv L, Liu C, Chen C, Yu X, Chen G, Shi Y, Qin F, Ou J, Qiu K, Li G (2016) Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget 7(22):32184–32199. https://doi.org/10.18632/oncotarget.8607
Shu Y, Xie B, Liang Z, Chen J (2018) Quercetin reverses the doxorubicin resistance of prostate cancer cells by downregulating the expression of c-met. Oncol Lett 15(2):2252–2258. https://doi.org/10.3892/ol.2017.7561
Atashpour S, Fouladdel S, Movahhed TK, Barzegar E, Ghahremani MH, Ostad SN, Azizi E (2015) Quercetin induces cell cycle arrest and apoptosis in CD133(+) cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran J Basic Med Sci 18(7):635–643
Lu X, Yang F, Chen D, Zhao Q, Chen D, Ping H, Xing N (2020) Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int J Biol Sci 16(7):1121–1134. https://doi.org/10.7150/ijbs.41686
Safi A, Heidarian E, Ahmadi R (2021) Quercetin synergistically enhances the anticancer efficacy of docetaxel through induction of apoptosis and modulation of PI3K/AKT, MAPK/ERK, and JAK/STAT3 signaling pathways in MDA-MB-231 breast cancer cell line. Int J Mol Cell Med 10(1):11–22. https://doi.org/10.22088/IJMCM.BUMS.10.1.11
Fujii K, Idogawa M, Suzuki N, Iwatsuki K, Kanekura T (2021) Functional depletion of HSP72 by siRNA and quercetin enhances vorinostat-induced apoptosis in an HSP72-overexpressing cutaneous T-cell lymphoma cell line, Hut78. Int J Mol Sci. https://doi.org/10.3390/ijms222011258
Wang Y, Yu H, Wang S, Gai C, Cui X, Xu Z, Li W, Zhang W (2021) Targeted delivery of quercetin by nanoparticles based on chitosan sensitizing paclitaxel-resistant lung cancer cells to paclitaxel. Mater Sci Eng C Mater Biol Appl 119:111442. https://doi.org/10.1016/j.msec.2020.111442
Molavi O, Narimani F, Asiaee F, Sharifi S, Tarhriz V, Shayanfar A, Hejazi M, Lai R (2017) Silibinin sensitizes chemo-resistant breast cancer cells to chemotherapy. Pharm Biol 55(1):729–739. https://doi.org/10.1080/13880209.2016.1270972
Catanzaro D, Gabbia D, Cocetta V, Biagi M, Ragazzi E, Montopoli M, Carrara M (2018) Silybin counteracts doxorubicin resistance by inhibiting GLUT1 expression. Fitoterapia 124:42–48. https://doi.org/10.1016/j.fitote.2017.10.007
Zhou L, Liu P, Chen B, Wang Y, Wang X, Chiriva Internati M, Wachtel MS, Frezza EE (2008) Silibinin restores paclitaxel sensitivity to paclitaxel-resistant human ovarian carcinoma cells. Anticancer Res 28(2A):1119–1127
Hussain SA, Marouf BH (2013) Silibinin improves the cytotoxicity of methotrexate in chemo resistant human rhabdomyosarcoma cell lines. Saudi Med J 34(11):1145–1150
Wang W, Chen D, Zhu K (2018) SOX2OT variant 7 contributes to the synergistic interaction between EGCG and Doxorubicin to kill osteosarcoma via autophagy and stemness inhibition. J Exp Clin Cancer Res 37(1):37. https://doi.org/10.1186/s13046-018-0689-3
Chen L, Ye HL, Zhang G, Yao WM, Chen XZ, Zhang FC, Liang G (2014) Autophagy inhibition contributes to the synergistic interaction between EGCG and doxorubicin to kill the hepatoma Hep3B cells. PLoS ONE 9(1):e85771. https://doi.org/10.1371/journal.pone.0085771
Stearns ME, Amatangelo MD, Varma D, Sell C, Goodyear SM (2010) Combination therapy with epigallocatechin-3-gallate and doxorubicin in human prostate tumor modeling studies: inhibition of metastatic tumor growth in severe combined immunodeficiency mice. Am J Pathol 177(6):3169–3179. https://doi.org/10.2353/ajpath.2010.100330
Giro-Perafita A, Rabionet M, Planas M, Feliu L, Ciurana J, Ruiz-Martinez S, Puig T (2019) EGCG-derivative G28 shows high efficacy inhibiting the mammosphere-forming capacity of sensitive and resistant TNBC models. Molecules. https://doi.org/10.3390/molecules24061027
Wen Y, Zhao RQ, Zhang YK, Gupta P, Fu LX, Tang AZ, Liu BM, Chen ZS, Yang DH, Liang G (2017) Effect of Y6, an epigallocatechin gallate derivative, on reversing doxorubicin drug resistance in human hepatocellular carcinoma cells. Oncotarget 8(18):29760–29770. https://doi.org/10.18632/oncotarget.15964
Toden S, Tran HM, Tovar-Camargo OA, Okugawa Y, Goel A (2016) Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 7(13):16158–16171. https://doi.org/10.18632/oncotarget.7567
Han JH, Kim M, Kim HJ, Jang SB, Bae SJ, Lee IK, Ryu D, Ha KT (2021) Targeting lactate dehydrogenase A with catechin resensitizes SNU620/5FU gastric cancer cells to 5-fluorouracil. Int J Mol Sci. https://doi.org/10.3390/ijms22105406
Mayr C, Wagner A, Neureiter D, Pichler M, Jakab M, Illig R, Berr F, Kiesslich T (2015) The green tea catechin epigallocatechin gallate induces cell cycle arrest and shows potential synergism with cisplatin in biliary tract cancer cells. BMC Complement Altern Med 15:194. https://doi.org/10.1186/s12906-015-0721-5
Tian M, Tian D, Qiao X, Li J, Zhang L (2019) Modulation of Myb-induced NF-kB -STAT3 signaling and resulting cisplatin resistance in ovarian cancer by dietary factors. J Cell Physiol 234(11):21126–21134. https://doi.org/10.1002/jcp.28715
Kumar BNP, Puvvada N, Rajput S, Sarkar S, Mahto MK, Yallapu MM, Pathak A, Emdad L, Das SK, Reis RL, Kundu SC, Fisher PB, Mandal M (2018) Targeting of EGFR, VEGFR2, and Akt by engineered dual drug encapsulated mesoporous silica-gold nanoclusters sensitizes tamoxifen-resistant breast cancer. Mol Pharm 15(7):2698–2713. https://doi.org/10.1021/acs.molpharmaceut.8b00218
Fu P, Du F, Chen W, Yao M, Lv K, Liu Y (2014) Tanshinone IIA blocks epithelial-mesenchymal transition through HIF-1alpha downregulation, reversing hypoxia-induced chemotherapy resistance in breast cancer cell lines. Oncol Rep 31(6):2561–2568. https://doi.org/10.3892/or.2014.3140
Li K, Liu W, Zhao Q, Wu C, Fan C, Lai H, Li S (2019) Combination of tanshinone IIA and doxorubicin possesses synergism and attenuation effects on doxorubicin in the treatment of breast cancer. Phytother Res 33(6):1658–1669. https://doi.org/10.1002/ptr.6353
Zu Y, Wang J, Ping W, Sun W (2018) Tan IIA inhibits H1299 cell viability through the MDM4IAP3 signaling pathway. Mol Med Rep 17(2):2384–2392. https://doi.org/10.3892/mmr.2017.8152
Jiao JW, Wen F (2011) Tanshinone IIA acts via p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and lung-resistance protein in cisplatin-resistant ovarian cancer cells. Oncol Rep 25(3):781–788. https://doi.org/10.3892/or.2010.1107
Zhong Y, Zhang F, Sun Z, Zhou W, Li ZY, You QD, Guo QL, Hu R (2013) Drug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense response. Mol Carcinog 52(10):824–834. https://doi.org/10.1002/mc.21921
Qian C, Wang Y, Zhong Y, Tang J, Zhang J, Li Z, Wang Q, Hu R (2014) Wogonin-enhanced reactive oxygen species-induced apoptosis and potentiated cytotoxic effects of chemotherapeutic agents by suppression Nrf2-mediated signaling in HepG2 cells. Free Radic Res 48(5):607–621. https://doi.org/10.3109/10715762.2014.897342
Kim EH, Jang H, Shin D, Baek SH, Roh JL (2016) Targeting Nrf2 with wogonin overcomes cisplatin resistance in head and neck cancer. Apoptosis 21(11):1265–1278. https://doi.org/10.1007/s10495-016-1284-8
Li S, Lei Y, Jia Y, Li N, Wink M, Ma Y (2011) Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine 19(1):83–87. https://doi.org/10.1016/j.phymed.2011.06.031
Morsy MA, El-Sheikh AAK, Ibrahim ARN, Khedr MA, Al-Taher AY (2018) In silico comparisons between natural inhibitors of ABCB1/P-glycoprotein to overcome doxorubicin-resistance in the NCI/ADR-RES cell line. Eur J Pharm Sci 112:87–94. https://doi.org/10.1016/j.ejps.2017.11.010
Xie Z, Wei Y, Xu J, Lei J, Yu J (2019) Alkaloids from Piper nigrum synergistically enhanced the effect of paclitaxel against paclitaxel-resistant cervical cancer cells through the downregulation of Mcl-1. J Agric Food Chem 67(18):5159–5168. https://doi.org/10.1021/acs.jafc.9b01320
Zhong T, Xu F, Xu J, Liu L, Chen Y (2015) Aldo-keto reductase 1C3 (AKR1C3) is associated with the doxorubicin resistance in human breast cancer via PTEN loss. Biomed Pharmacother 69:317–325. https://doi.org/10.1016/j.biopha.2014.12.022
Bains OS, Grigliatti TA, Reid RE, Riggs KW (2010) Naturally occurring variants of human aldo-keto reductases with reduced in vitro metabolism of daunorubicin and doxorubicin. J Pharmacol Exp Ther 335(3):533–545. https://doi.org/10.1124/jpet.110.173179
Heibein AD, Guo B, Sprowl JA, Maclean DA, Parissenti AM (2012) Role of aldo-keto reductases and other doxorubicin pharmacokinetic genes in doxorubicin resistance, DNA binding, and subcellular localization. BMC Cancer 12:381. https://doi.org/10.1186/1471-2407-12-381
Bains OS, Szeitz A, Lubieniecka JM, Cragg GE, Grigliatti TA, Riggs KW, Reid RE (2013) A correlation between cytotoxicity and reductase-mediated metabolism in cell lines treated with doxorubicin and daunorubicin. J Pharmacol Exp Ther 347(2):375–387. https://doi.org/10.1124/jpet.113.206805
Jo A, Choi TG, Jo YH, Jyothi KR, Nguyen MN, Kim JH, Lim S, Shahid M, Akter S, Lee S, Lee KH, Kim W, Cho H, Lee J, Shokat KM, Yoon KS, Kang I, Ha J, Kim SS (2017) Inhibition of carbonyl reductase 1 safely improves the efficacy of doxorubicin in breast cancer treatment. Antioxid Redox Signal 26(2):70–83. https://doi.org/10.1089/ars.2015.6457
Shan L, Gao G, Wang W, Tang W, Wang Z, Yang Z, Fan W, Zhu G, Zhai K, Jacobson O, Dai Y, Chen X (2019) Self-assembled green tea polyphenol-based coordination nanomaterials to improve chemotherapy efficacy by inhibition of carbonyl reductase 1. Biomaterials 210:62–69. https://doi.org/10.1016/j.biomaterials.2019.04.032
Tao NN, Zhou HZ, Tang H, Cai XF, Zhang WL, Ren JH, Zhou L, Chen X, Chen K, Li WY, Liu B, Yang QX, Cheng ST, Huang LX, Huang AL, Chen J (2016) Sirtuin 3 enhanced drug sensitivity of human hepatoma cells through glutathione S-transferase pi 1/JNK signaling pathway. Oncotarget 7(31):50117–50130. https://doi.org/10.18632/oncotarget.10319
Kim D, Choi BH, Ryoo IG, Kwak MK (2018) High NRF2 level mediates cancer stem cell-like properties of aldehyde dehydrogenase (ALDH)-high ovarian cancer cells: inhibitory role of all-trans retinoic acid in ALDH/NRF2 signaling. Cell Death Dis 9(9):896. https://doi.org/10.1038/s41419-018-0903-4
Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N (2014) Apoptosis and molecular targeting therapy in cancer. Biomed Res Int 2014:150845. https://doi.org/10.1155/2014/150845
Yang MC, Lin RW, Huang SB, Huang SY, Chen WJ, Wang S, Hong YR, Wang C (2016) Bim directly antagonizes Bcl-xl in doxorubicin-induced prostate cancer cell apoptosis independently of p53. Cell Cycle 15(3):394–402. https://doi.org/10.1080/15384101.2015.1127470
Baranski Z, de Jong Y, Ilkova T, Peterse EF, Cleton-Jansen AM, van de Water B, Hogendoorn PC, Bovee JV, Danen EH (2015) Pharmacological inhibition of Bcl-xL sensitizes osteosarcoma to doxorubicin. Oncotarget 6(34):36113-36125. https://doi.org/10.18632/oncotarget.5333
Song C, Ge Z, Ding Y, Tan BH, Desai D, Gowda K, Amin S, Gowda R, Robertson GP, Yue F, Huang S, Spiegelman V, Payne JL, Reeves ME, Gurel Z, Iyer S, Dhanyamraju PK, Xiang M, Kawasawa YI, Cury NM, Yunes JA, McGrath M, Schramm J, Su R, Yang Y, Zhao Z, Lyu X, Muschen M, Payne KJ, Gowda C, Dovat S (2020) IKAROS and CK2 regulate expression of BCL-XL and chemosensitivity in high-risk B-cell acute lymphoblastic leukemia. Blood 136(13):1520–1534. https://doi.org/10.1182/blood.2019002655
Sims JT, Ganguly SS, Bennett H, Friend JW, Tepe J, Plattner R (2013) Imatinib reverses doxorubicin resistance by affecting activation of STAT3-dependent NF-kappaB and HSP27/p38/AKT pathways and by inhibiting ABCB1. PLoS ONE 8(1):e55509. https://doi.org/10.1371/journal.pone.0055509
Shi Y, Wang SY, Yao M, Sai WL, Wu W, Yang JL, Cai Y, Zheng WJ, Yao DF (2015) Chemosensitization of HepG2 cells by suppression of NF-kappaB/p65 gene transcription with specific-siRNA. World J Gastroenterol 21(45):12814–12821. https://doi.org/10.3748/wjg.v21.i45.12814
Tarasewicz E, Hamdan R, Straehla J, Hardy A, Nunez O, Zelivianski S, Dokic D, Jeruss JS (2014) CDK4 inhibition and doxorubicin mediate breast cancer cell apoptosis through Smad3 and survivin. Cancer Biol Ther 15(10):1301–1311. https://doi.org/10.4161/cbt.29693
Jabbour-Leung NA, Chen X, Bui T, Jiang Y, Yang D, Vijayaraghavan S, McArthur MJ, Hunt KK, Keyomarsi K (2016) Sequential combination therapy of CDK inhibition and doxorubicin is synthetically lethal in p53-mutant triple-negative breast cancer. Mol Cancer Ther 15(4):593–607. https://doi.org/10.1158/1535-7163.MCT-15-0519
Lu J, Guan S, Zhao Y, Yu Y, Wang Y, Shi Y, Mao X, Yang KL, Sun W, Xu X, Yi JS, Yang T, Yang J, Nuchtern JG (2016) Novel MDM2 inhibitor SAR405838 (MI-773) induces p53-mediated apoptosis in neuroblastoma. Oncotarget 7(50):82757–82769. https://doi.org/10.18632/oncotarget.12634
Zu Y, Yang Z, Tang S, Han Y, Ma J (2014) Effects of P-glycoprotein and its inhibitors on apoptosis in K562 cells. Molecules 19(9):13061–13075. https://doi.org/10.3390/molecules190913061
Faversani A, Vaira V, Moro GP, Tosi D, Lopergolo A, Schultz DC, Rivadeneira D, Altieri DC, Bosari S (2014) Survivin family proteins as novel molecular determinants of doxorubicin resistance in organotypic human breast tumors. Breast Cancer Res 16(3):R55. https://doi.org/10.1186/bcr3666
Liu YY, Patwardhan GA, Bhinge K, Gupta V, Gu X, Jazwinski SM (2011) Suppression of glucosylceramide synthase restores p53-dependent apoptosis in mutant p53 cancer cells. Cancer Res 71(6):2276–2285. https://doi.org/10.1158/0008-5472.CAN-10-3107
Zhang X, Wu X, Su P, Gao Y, Meng B, Sun Y, Li L, Zhou Z, Zhou G (2012) Doxorubicin influences the expression of glucosylceramide synthase in invasive ductal breast cancer. PLoS ONE 7(11):e48492. https://doi.org/10.1371/journal.pone.0048492
Salaroglio IC, Gazzano E, Abdullrahman A, Mungo E, Castella B, Abd-Elrahman G, Massaia M, Donadelli M, Rubinstein M, Riganti C, Kopecka J (2018) Increasing intratumor C/EBP-beta LIP and nitric oxide levels overcome resistance to doxorubicin in triple negative breast cancer. J Exp Clin Cancer Res 37(1):286. https://doi.org/10.1186/s13046-018-0967-0
Cheriyath V, Kuhns MA, Kalaycio ME, Borden EC (2011) Potentiation of apoptosis by histone deacetylase inhibitors and doxorubicin combination: cytoplasmic cathepsin B as a mediator of apoptosis in multiple myeloma. Br J Cancer 104(6):957–967. https://doi.org/10.1038/bjc.2011.42
Turner JG, Dawson JL, Grant S, Shain KH, Dalton WS, Dai Y, Meads M, Baz R, Kauffman M, Shacham S, Sullivan DM (2016) Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase II inhibitors. J Hematol Oncol 9(1):73. https://doi.org/10.1186/s13045-016-0304-z
Huang Y, Jiang D, Sui M, Wang X, Fan W (2017) Fulvestrant reverses doxorubicin resistance in multidrug-resistant breast cell lines independent of estrogen receptor expression. Oncol Rep 37(2):705–712. https://doi.org/10.3892/or.2016.5315
Ku JM, Kim SR, Hong SH, Choi HS, Seo HS, Shin YC, Ko SG (2015) Cucurbitacin D induces cell cycle arrest and apoptosis by inhibiting STAT3 and NF-kappaB signaling in doxorubicin-resistant human breast carcinoma (MCF7/ADR) cells. Mol Cell Biochem 409(1–2):33–43. https://doi.org/10.1007/s11010-015-2509-9
Lin Y, Kang T, Zhou BP (2014) Doxorubicin enhances Snail/LSD1-mediated PTEN suppression in a PARP1-dependent manner. Cell Cycle 13(11):1708–1716. https://doi.org/10.4161/cc.28619
Yang J, Yu H, Shen M, Wei W, Xia L, Zhao P (2014) N1-guanyl-1,7-diaminoheptane sensitizes bladder cancer cells to doxorubicin by preventing epithelial-mesenchymal transition through inhibition of eukaryotic translation initiation factor 5A2 activation. Cancer Sci 105(2):219–227. https://doi.org/10.1111/cas.12328
Zhou Y, Liang C, Xue F, Chen W, Zhi X, Feng X, Bai X, Liang T (2015) Salinomycin decreases doxorubicin resistance in hepatocellular carcinoma cells by inhibiting the beta-catenin/TCF complex association via FOXO3a activation. Oncotarget 6(12):10350–10365. https://doi.org/10.18632/oncotarget.3585
Yao C, Wu S, Li D, Ding H, Wang Z, Yang Y, Yan S, Gu Z (2012) Co-administration phenoxodiol with doxorubicin synergistically inhibit the activity of sphingosine kinase-1 (SphK1), a potential oncogene of osteosarcoma, to suppress osteosarcoma cell growth both in vivo and in vitro. Mol Oncol 6(4):392–404. https://doi.org/10.1016/j.molonc.2012.04.002
Kumar AP, Loo SY, Shin SW, Tan TZ, Eng CB, Singh R, Putti TC, Ong CW, Salto-Tellez M, Goh BC, Park JI, Thiery JP, Pervaiz S, Clement MV (2014) Manganese superoxide dismutase is a promising target for enhancing chemosensitivity of basal-like breast carcinoma. Antioxid Redox Signal 20(15):2326–2346. https://doi.org/10.1089/ars.2013.5295
Sonowal H, Pal PB, Wen JJ, Awasthi S, Ramana KV, Srivastava SK (2017) Aldose reductase inhibitor increases doxorubicin-sensitivity of colon cancer cells and decreases cardiotoxicity. Sci Rep 7(1):3182. https://doi.org/10.1038/s41598-017-03284-w
Westhoff MA, Faham N, Marx D, Nonnenmacher L, Jennewein C, Enzenmuller S, Gonzalez P, Fulda S, Debatin KM (2013) Sequential dosing in chemosensitization: targeting the PI3K/Akt/mTOR pathway in neuroblastoma. PLoS ONE 8(12):e83128. https://doi.org/10.1371/journal.pone.0083128
Li YT, Qian XJ, Yu Y, Li ZH, Wu RY, Ji J, Jiao L, Li X, Kong PF, Chen WD, Feng GK, Deng R, Zhu XF (2015) EGFR tyrosine kinase inhibitors promote pro-caspase-8 dimerization that sensitizes cancer cells to DNA-damaging therapy. Oncotarget 6(19):17491–17500. https://doi.org/10.18632/oncotarget.3959
Friesen C, Hormann I, Roscher M, Fichtner I, Alt A, Hilger R, Debatin KM, Miltner E (2014) Opioid receptor activation triggering downregulation of cAMP improves effectiveness of anti-cancer drugs in treatment of glioblastoma. Cell Cycle 13(10):1560–1570. https://doi.org/10.4161/cc.28493
Li ZL, Chen C, Yang Y, Wang C, Yang T, Yang X, Liu SC (2015) Gamma secretase inhibitor enhances sensitivity to doxorubicin in MDA-MB-231 cells. Int J Clin Exp Pathol 8(5):4378–4387
Durrant DE, Das A, Dyer S, Tavallai S, Dent P, Kukreja RC (2015) Targeted inhibition of phosphoinositide 3-kinase/mammalian target of rapamycin sensitizes pancreatic cancer cells to doxorubicin without exacerbating cardiac toxicity. Mol Pharmacol 88(3):512–523. https://doi.org/10.1124/mol.115.099143
Yndestad S, Austreid E, Svanberg IR, Knappskog S, Lonning PE, Eikesdal HP (2017) Activation of Akt characterizes estrogen receptor positive human breast cancers which respond to anthracyclines. Oncotarget 8(25):41227–41241. https://doi.org/10.18632/oncotarget.17167
Kang JH, Song KH, Jeong KC, Kim S, Choi C, Lee CH, Oh SH (2011) Involvement of Cox-2 in the metastatic potential of chemotherapy-resistant breast cancer cells. BMC Cancer 11:334. https://doi.org/10.1186/1471-2407-11-334
Carlisi D, De Blasio A, Drago-Ferrante R, Di Fiore R, Buttitta G, Morreale M, Scerri C, Vento R, Tesoriere G (2017) Parthenolide prevents resistance of MDA-MB231 cells to doxorubicin and mitoxantrone: the role of Nrf2. Cell Death Discov 3:17078. https://doi.org/10.1038/cddiscovery.2017.78
Fang LM, Li B, Guan JJ, Xu HD, Shen GH, Gao QG, Qin ZH (2017) Transcription factor EB is involved in autophagy-mediated chemoresistance to doxorubicin in human cancer cells. Acta Pharmacol Sin 38(9):1305–1316. https://doi.org/10.1038/aps.2017.25
Liang L, Fu J, Wang S, Cen H, Zhang L, Mandukhail SR, Du L, Wu Q, Zhang P, Yu X (2020) MiR-142-3p enhances chemosensitivity of breast cancer cells and inhibits autophagy by targeting HMGB1. Acta Pharm Sin B 10(6):1036–1046. https://doi.org/10.1016/j.apsb.2019.11.009
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435(7043):834–838. https://doi.org/10.1038/nature03702
Bader AG, Brown D, Winkler M (2010) The promise of microRNA replacement therapy. Cancer Res 70(18):7027–7030. https://doi.org/10.1158/0008-5472.CAN-10-2010
Dana H, Chalbatani GM, Mahmoodzadeh H, Karimloo R, Rezaiean O, Moradzadeh A, Mehmandoost N, Moazzen F, Mazraeh A, Marmari V, Ebrahimi M, Rashno MM, Abadi SJ, Gharagouzlo E (2017) Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci 13(2):48–57
Carthew RW, Sontheimer EJ (2009) Origins and Mechanisms of miRNAs and siRNAs. Cell 136(4):642–655. https://doi.org/10.1016/j.cell.2009.01.035
van Rooij E, Purcell AL, Levin AA (2012) Developing microRNA therapeutics. Circ Res 110(3):496–507. https://doi.org/10.1161/CIRCRESAHA.111.247916
Shi X, Valizadeh A, Mir SM, Asemi Z, Karimian A, Majidina M, Safa A, Yosefi B (2020) miRNA-29a reverses P-glycoprotein-mediated drug resistance and inhibits proliferation via up-regulation of PTEN in colon cancer cells. Eur J Pharmacol 880:173138. https://doi.org/10.1016/j.ejphar.2020.173138
Zhong S, Li W, Chen Z, Xu J, Zhao J (2013) MiR-222 and miR-29a contribute to the drug-resistance of breast cancer cells. Gene 531(1):8–14. https://doi.org/10.1016/j.gene.2013.08.062
Park EY, Chang E, Lee EJ, Lee HW, Kang HG, Chun KH, Woo YM, Kong HK, Ko JY, Suzuki H, Song E, Park JH (2014) Targeting of miR34a-NOTCH1 axis reduced breast cancer stemness and chemoresistance. Cancer Res 74(24):7573–7582. https://doi.org/10.1158/0008-5472.CAN-14-1140
Zhou Y, Zhao RH, Tseng KF, Li KP, Lu ZG, Liu Y, Han K, Gan ZH, Lin SC, Hu HY, Min DL (2016) Sirolimus induces apoptosis and reverses multidrug resistance in human osteosarcoma cells in vitro via increasing microRNA-34b expression. Acta Pharmacol Sin 37(4):519–529. https://doi.org/10.1038/aps.2015.153
Li MM, Addepalli B, Tu MJ, Chen QX, Wang WP, Limbach PA, LaSalle JM, Zeng S, Huang M, Yu AM (2015) Chimeric microRNA-1291 biosynthesized efficiently in Escherichia coli is effective to reduce target gene expression in human carcinoma cells and improve chemosensitivity. Drug Metab Dispos 43(7):1129–1136. https://doi.org/10.1124/dmd.115.064493
Jin Y, Wang H, Zhu Y, Feng H, Wang G, Wang S (2020) miR-199a-5p is involved in doxorubicin resistance of non-small cell lung cancer (NSCLC) cells. Eur J Pharmacol 878:173105. https://doi.org/10.1016/j.ejphar.2020.173105
Henry JC, Park JK, Jiang J, Kim JH, Nagorney DM, Roberts LR, Banerjee S, Schmittgen TD (2010) miR-199a-3p targets CD44 and reduces proliferation of CD44 positive hepatocellular carcinoma cell lines. Biochem Biophys Res Commun 403(1):120–125. https://doi.org/10.1016/j.bbrc.2010.10.130
Lou G, Chen L, Xia C, Wang W, Qi J, Li A, Zhao L, Chen Z, Zheng M, Liu Y (2020) MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res 39(1):4. https://doi.org/10.1186/s13046-019-1512-5
Takwi AA, Wang YM, Wu J, Michaelis M, Cinatl J, Chen T (2014) miR-137 regulates the constitutive androstane receptor and modulates doxorubicin sensitivity in parental and doxorubicin-resistant neuroblastoma cells. Oncogene 33(28):3717–3729. https://doi.org/10.1038/onc.2013.330
Wang ZC, Huang FZ, Xu HB, Sun JC, Wang CF (2019) MicroRNA-137 inhibits autophagy and chemosensitizes pancreatic cancer cells by targeting ATG5. Int J Biochem Cell Biol 111:63–71. https://doi.org/10.1016/j.biocel.2019.01.020
Zhu X, Li Y, Shen H, Li H, Long L, Hui L, Xu W (2013) miR-137 restoration sensitizes multidrug-resistant MCF-7/ADM cells to anticancer agents by targeting YB-1. Acta Biochim Biophys Sin (Shanghai) 45(2):80–86. https://doi.org/10.1093/abbs/gms099
Du F, Yu L, Wu Y, Wang S, Yao J, Zheng X, Xie S, Zhang S, Lu X, Liu Y, Chen W (2019) miR-137 alleviates doxorubicin resistance in breast cancer through inhibition of epithelial-mesenchymal transition by targeting DUSP4. Cell Death Dis 10(12):922. https://doi.org/10.1038/s41419-019-2164-2
Lai J, Yang H, Zhu Y, Ruan M, Huang Y, Zhang Q (2019) MiR-7-5p-mediated downregulation of PARP1 impacts DNA homologous recombination repair and resistance to doxorubicin in small cell lung cancer. BMC Cancer 19(1):602. https://doi.org/10.1186/s12885-019-5798-7
He DX, Gu XT, Li YR, Jiang L, Jin J, Ma X (2014) Methylation-regulated miR-149 modulates chemoresistance by targeting GlcNAc N-deacetylase/N-sulfotransferase-1 in human breast cancer. FEBS J 281(20):4718–4730. https://doi.org/10.1111/febs.13012
Jin F, Wang Y, Li M, Zhu Y, Liang H, Wang C, Wang F, Zhang CY, Zen K, Li L (2017) MiR-26 enhances chemosensitivity and promotes apoptosis of hepatocellular carcinoma cells through inhibiting autophagy. Cell Death Dis 8(1):e2540. https://doi.org/10.1038/cddis.2016.461
Qu J, Zhao L, Zhang P, Wang J, Xu N, Mi W, Jiang X, Zhang C, Qu J (2015) MicroRNA-195 chemosensitizes colon cancer cells to the chemotherapeutic drug doxorubicin by targeting the first binding site of BCL2L2 mRNA. J Cell Physiol 230(3):535–545. https://doi.org/10.1002/jcp.24366
Yang G, Wu D, Zhu J, Jiang O, Shi Q, Tian J, Weng Y (2013) Upregulation of miR-195 increases the sensitivity of breast cancer cells to Adriamycin treatment through inhibition of Raf-1. Oncol Rep 30(2):877–889. https://doi.org/10.3892/or.2013.2532
Yi H, Liu L, Sheng N, Li P, Pan H, Cai L, Ma Y (2016) Synergistic therapy of doxorubicin and miR-129-5p with self-cross-linked bioreducible polypeptide nanoparticles reverses multidrug resistance in cancer cells. Biomacromol 17(5):1737–1747. https://doi.org/10.1021/acs.biomac.6b00141
Zeng H, Wang L, Wang J, Chen T, Li H, Zhang K, Chen J, Zhen S, Tuluhong D, Li J, Wang S (2018) microRNA-129-5p suppresses Adriamycin resistance in breast cancer by targeting SOX2. Arch Biochem Biophys 651:52–60. https://doi.org/10.1016/j.abb.2018.05.018
Mi H, Wang X, Wang F, Li L, Zhu M, Wang N, Xiong Y, Gu Y (2018) miR-381 induces sensitivity of breast cancer cells to doxorubicin by inactivation of MAPK signaling via FYN. Eur J Pharmacol 839:66–75. https://doi.org/10.1016/j.ejphar.2018.09.024
Wang Y, Zeng L, Liang C, Zan R, Ji W, Zhang Z, Wei Y, Tu S, Dong Y (2019) Integrated analysis of transcriptome-wide m(6)A methylome of osteosarcoma stem cells enriched by chemotherapy. Epigenomics 11(15):1693–1715. https://doi.org/10.2217/epi-2019-0262
Zhang R, Li SW, Liu L, Yang J, Huang G, Sang Y (2020) TRIM11 facilitates chemoresistance in nasopharyngeal carcinoma by activating the beta-catenin/ABCC9 axis via p62-selective autophagic degradation of Daple. Oncogenesis 9(5):45. https://doi.org/10.1038/s41389-020-0229-9
Shi Y, Fan S, Wu M, Zuo Z, Li X, Jiang L, Shen Q, Xu P, Zeng L, Zhou Y, Huang Y, Yang Z, Zhou J, Gao J, Zhou H, Xu S, Ji H, Shi P, Wu DD, Yang C, Chen Y (2019) YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat Commun 10(1):4892. https://doi.org/10.1038/s41467-019-12801-6
Tang B, Yang Y, Kang M, Wang Y, Wang Y, Bi Y, He S, Shimamoto F (2020) m(6)A demethylase ALKBH5 inhibits pancreatic cancer tumorigenesis by decreasing WIF-1 RNA methylation and mediating Wnt signaling. Mol Cancer 19(1):3. https://doi.org/10.1186/s12943-019-1128-6
Fukumoto T, Zhu H, Nacarelli T, Karakashev S, Fatkhutdinov N, Wu S, Liu P, Kossenkov AV, Showe LC, Jean S, Zhang L, Zhang R (2019) N(6)-methylation of adenosine of FZD10 mRNA contributes to PARP inhibitor resistance. Cancer Res 79(11):2812–2820. https://doi.org/10.1158/0008-5472.CAN-18-3592
Taketo K, Konno M, Asai A, Koseki J, Toratani M, Satoh T, Doki Y, Mori M, Ishii H, Ogawa K (2018) The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int J Oncol 52(2):621–629. https://doi.org/10.3892/ijo.2017.4219
Pan X, Hong X, Li S, Meng P, Xiao F (2021) METTL3 promotes adriamycin resistance in MCF-7 breast cancer cells by accelerating pri-microRNA-221-3p maturation in a m6A-dependent manner. Exp Mol Med 53(1):91–102. https://doi.org/10.1038/s12276-020-00510-w
Wu Y, Wang Z, Han L, Guo Z, Yan B, Guo L, Zhao H, Wei M, Hou N, Ye J, Wang Z, Shi C, Liu S, Chen C, Chen S, Wang T, Yi J, Zhou J, Yao L, Zhou W, Ling R, Zhang J (2022) PRMT5 regulates RNA m6A demethylation for doxorubicin sensitivity in breast cancer. Mol Ther. https://doi.org/10.1016/j.ymthe.2022.03.003
Yang Z, Xiao T, Li Z, Zhang J, Chen S (2022) Novel chemicals derived from tadalafil exhibit PRMT5 inhibition and promising activities against breast cancer. Int J Mol Sci. https://doi.org/10.3390/ijms23094806
Lam JK, Chow MY, Zhang Y, Leung SW (2015) siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids 4:e252. https://doi.org/10.1038/mtna.2015.23
Alshaer W, Alqudah DA, Wehaibi S, Abuarqoub D, Zihlif M, Hatmal MM, Awidi A (2019) Downregulation of STAT3, beta-Catenin, and Notch-1 by single and combinations of siRNA treatment enhance chemosensitivity of wild type and doxorubicin resistant MCF7 breast cancer cells to doxorubicin. Int J Mol Sci. https://doi.org/10.3390/ijms20153696
Zhou W, Tan W, Huang X, Yu HG (2018) Doxorubicin combined with Notch1-targeting siRNA for the treatment of gastric cancer. Oncol Lett 16(3):2805–2812. https://doi.org/10.3892/ol.2018.9039
Perez J, Bardin C, Rigal C, Anthony B, Rousseau R, Dutour A (2011) Anti-MDR1 siRNA restores chemosensitivity in chemoresistant breast carcinoma and osteosarcoma cell lines. Anticancer Res 31(9):2813–2820
Wang D, Xu X, Zhang K, Sun B, Wang L, Meng L, Liu Q, Zheng C, Yang B, Sun H (2018) Codelivery of doxorubicin and MDR1-siRNA by mesoporous silica nanoparticles-polymerpolyethylenimine to improve oral squamous carcinoma treatment. Int J Nanomed 13:187–198. https://doi.org/10.2147/IJN.S150610
Shen J, Wang Q, Hu Q, Li Y, Tang G, Chu PK (2014) Restoration of chemosensitivity by multifunctional micelles mediated by P-gp siRNA to reverse MDR. Biomaterials 35(30):8621–8634. https://doi.org/10.1016/j.biomaterials.2014.06.035
Shi Z, Yang WM, Chen LP, Yang DH, Zhou Q, Zhu J, Chen JJ, Huang RC, Chen ZS, Huang RP (2012) Enhanced chemosensitization in multidrug-resistant human breast cancer cells by inhibition of IL-6 and IL-8 production. Breast Cancer Res Treat 135(3):737–747. https://doi.org/10.1007/s10549-012-2196-0
Chen Y, Liu J, Lv P, Gao J, Wang M, Wang Y (2018) IL-6 is involved in malignancy and doxorubicin sensitivity of renal carcinoma cells. Cell Adh Migr 12(1):28–36. https://doi.org/10.1080/19336918.2017.1307482
Chinh Chung D, Thanh Long L, Nghia Son H, Tri Bao L, Minh Si D, le Dong V (2015) Downregulation of vascular endothelial growth factor enhances chemosensitivity by induction of apoptosis in hepatocellular carcinoma cells. Cell J 17(2):273–287. https://doi.org/10.22074/cellj.2016.3730
Tiash S, Chua MJ, Chowdhury EH (2016) Knockdown of ROS1 gene sensitizes breast tumor growth to doxorubicin in a syngeneic mouse model. Int J Oncol 48(6):2359–2366. https://doi.org/10.3892/ijo.2016.3452
Liu Y, Du F, Chen W, Yao M, Lv K, Fu P (2013) Knockdown of dual specificity phosphatase 4 enhances the chemosensitivity of MCF-7 and MCF-7/ADR breast cancer cells to doxorubicin. Exp Cell Res 319(20):3140–3149. https://doi.org/10.1016/j.yexcr.2013.08.023
Jiao XL, Zhao C, Niu M, Chen D (2013) Downregulation of CD24 inhibits invasive growth, facilitates apoptosis and enhances chemosensitivity in gastric cancer AGS cells. Eur Rev Med Pharmacol Sci 17(13):1709–1715
Wang NS, Wei M, Ma WL, Meng W, Zheng WL (2014) Knockdown of CD44 enhances chemosensitivity of acute myeloid leukemia cells to ADM and Ara-C. Tumour Biol 35(4):3933–3940. https://doi.org/10.1007/s13277-013-1523-3
Vahidian F, Safarzadeh E, Mohammadi A, Najjary S, Mansoori B, Majidi J, Babaloo Z, Aghanejad A, Shadbad MA, Mokhtarzadeh A, Baradaran B (2020) siRNA-mediated silencing of CD44 delivered by Jet Pei enhanced Doxorubicin chemo sensitivity and altered miRNA expression in human breast cancer cell line (MDA-MB468). Mol Biol Rep 47(12):9541–9551. https://doi.org/10.1007/s11033-020-05952-z
Wang Y, Wang F, Liu Y, Xu S, Shen Y, Feng N, Guo S (2018) Glutathione detonated and pH responsive nano-clusters of Au nanorods with a high dose of DOX for treatment of multidrug resistant cancer. Acta Biomater 75:334–345. https://doi.org/10.1016/j.actbio.2018.06.012
He H, Liu L, Zhang S, Zheng M, Ma A, Chen Z, Pan H, Zhou H, Liang R, Cai L (2020) Smart gold nanocages for mild heat-triggered drug release and breaking chemoresistance. J Control Release 323:387–397. https://doi.org/10.1016/j.jconrel.2020.04.029
Zhang Z, Wang Y, Xu S, Yu Y, Hussain A, Shen Y, Guo S (2017) Photothermal gold nanocages filled with temperature sensitive tetradecanol and encapsulated with glutathione responsive polycurcumin for controlled DOX delivery to maximize anti-MDR tumor effects. J Mater Chem B 5(27):5464–5472. https://doi.org/10.1039/c7tb01253e
Mao HL, Qian F, Li S, Shen JW, Ye CK, Hua L, Zhang LZ, Wu DM, Lu J, Yu RT, Liu HM (2019) Delivery of doxorubicin from hyaluronic acid-modified glutathione-responsive ferrocene micelles for combination cancer therapy. Mol Pharm 16(3):987–994. https://doi.org/10.1021/acs.molpharmaceut.8b00862
Liu J, Chen C, Wei T, Gayet O, Loncle C, Borge L, Dusetti N, Ma X, Marson D, Laurini E, Pricl S, Gu Z, Iovanna J, Peng J, Liang XJ (2021) Dendrimeric nanosystem consistently circumvents heterogeneous drug response and resistance in pancreatic cancer. Exploration 1(1):21–34. https://doi.org/10.1002/EXP.20210003
Fan J, He Q, Liu Y, Zhang F, Yang X, Wang Z, Lu N, Fan W, Lin L, Niu G, He N, Song J, Chen X (2016) Light-responsive biodegradable nanomedicine overcomes multidrug resistance via NO-enhanced chemosensitization. ACS Appl Mater Interfaces 8(22):13804–13811. https://doi.org/10.1021/acsami.6b03737
Liu HN, Guo NN, Guo WW, Huang-Fu MY, Vakili MR, Chen JJ, Xu WH, Wei QC, Han M, Lavasanifar A, Gao JQ (2018) Delivery of mitochondriotropic doxorubicin derivatives using self-assembling hyaluronic acid nanocarriers in doxorubicin-resistant breast cancer. Acta Pharmacol Sin 39(10):1681–1692. https://doi.org/10.1038/aps.2018.9
Wang Q, Zou C, Wang L, Gao X, Wu J, Tan S, Wu G (2019) Doxorubicin and adjudin co-loaded pH-sensitive nanoparticles for the treatment of drug-resistant cancer. Acta Biomater 94:469–481. https://doi.org/10.1016/j.actbio.2019.05.061
Wu W, Chen M, Luo T, Fan Y, Zhang J, Zhang Y, Zhang Q, Sapin-Minet A, Gaucher C, Xia X (2020) ROS and GSH-responsive S-nitrosoglutathione functionalized polymeric nanoparticles to overcome multidrug resistance in cancer. Acta Biomater 103:259–271. https://doi.org/10.1016/j.actbio.2019.12.016
Ma YC, Wang JX, Tao W, Sun CY, Wang YC, Li DD, Fan F, Qian HS, Yang XZ (2015) Redox-responsive polyphosphoester-based micellar nanomedicines for overriding chemoresistance in breast cancer cells. ACS Appl Mater Interfaces 7(47):26315–26325. https://doi.org/10.1021/acsami.5b09195
Wang Y, Yi S, Sun L, Huang Y, Lenaghan SC, Zhang M (2014) Doxorubicin-loaded cyclic peptide nanotube bundles overcome chemoresistance in breast cancer cells. J Biomed Nanotechnol 10(3):445–454. https://doi.org/10.1166/jbn.2014.1724
Zhang X, Chen W, Bai J, Jin L, Kang X, Zhang H, Wang Z (2020) Pluronic P123 modified nano micelles loaded with doxorubicin enhanced tumor-suppressing effect on drug-resistant breast cancer cells. Aging 12(9):8289–8300. https://doi.org/10.18632/aging.103138
Wen ZM, Jie J, Zhang Y, Liu H, Peng LP (2017) A self-assembled polyjuglanin nanoparticle loaded with doxorubicin and anti-Kras siRNA for attenuating multidrug resistance in human lung cancer. Biochem Biophys Res Commun 493(4):1430–1437. https://doi.org/10.1016/j.bbrc.2017.09.132
Rastegar R, Akbari Javar H, Khoobi M, Dehghan Kelishadi P, Hossein Yousefi G, Doosti M, Hossien Ghahremani M, Shariftabrizi A, Imanparast F, Gholibeglu E, Gholami M (2018) Evaluation of a novel biocompatible magnetic nanomedicine based on beta-cyclodextrin, loaded doxorubicin-curcumin for overcoming chemoresistance in breast cancer. Artif Cells Nanomed Biotechnol 46(sup2):207–216. https://doi.org/10.1080/21691401.2018.1453829
Tannock IF (2015) Cancer: resistance through repopulation. Nature 517(7533):152–153. https://doi.org/10.1038/nature14075
Ramos A, Sadeghi S, Tabatabaeian H (2021) Battling chemoresistance in cancer: root causes and strategies to uproot them. Int J Mol Sci. https://doi.org/10.3390/ijms22179451
Acknowledgements
The authors would like to acknowledge Department of Science and Technology- INSPIRE Fellowship (IF180660), Ministry of Science and Technology, Government of India, for providing financial support to SS. The authors would also like to acknowledge the Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Kattankulathur-603203, Tamil Nadu, India, for the infrastructural support.
Funding
The authors would like to acknowledge the Department of Science and Technology-Inspire Fellowship (IF180660), Ministry of Science and Technology, Government of India, for providing financial support to SS.
Author information
Authors and Affiliations
Contributions
Manuscript preparation was performed by NS, SS, SG and SH. Designing, editing, and revisions were performed by NS and SS.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sritharan, S., Guha, S., Hazarika, S. et al. Meta analysis of bioactive compounds, miRNA, siRNA and cell death regulators as sensitizers to doxorubicin induced chemoresistance. Apoptosis 27, 622–646 (2022). https://doi.org/10.1007/s10495-022-01742-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-022-01742-z