Abstract
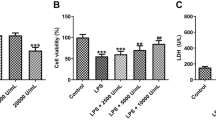
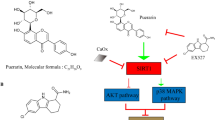
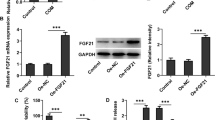
Aristolochic acid nephropathy remains a leading cause of chronic kidney disease (CKD), however few treatment strategies exist. Emerging evidence has shown that H2 relaxin (RLX) possesses powerful antifibrosis and anti-apoptotic properties, therefore we aimed to investigate whether H2 relaxin can be employed to reduce AA-induced cell apoptosis. Human proximal tubular epithelial (HK-2) cells exposed to AA-I were treated with or without administration of H2 RLX. Cell viability was examined using the WST-8 assay. Apoptotic morphologic alterations were observed using the Hoechst 33342 staining method. Apoptosis was detected using flow cytometry. The expression of caspase 3, caspase 8, caspase 9, ERK1/2, Bax, Bcl-2, and Akt proteins was determined by Western blot. Co-treatment with RLX reversed the increased apoptosis observed in the AA-I only treated group. RLX restored expression of phosphorylated Akt which found to be decreased in the AA-I only treated cells. RLX co-treatment led to a decrease in the Bax/Bcl-2 ratio as well as the cleaved form of caspase-3 compared to the AA-I only treated cells. This anti-apoptotic effect of RLX was attenuated by co-administration of the Akt inhibitor LY294002. The present study demonstrated H2 RLX can decrease AA-I induced apoptosis through activation of the PI3K/Akt signaling pathway.







Similar content being viewed by others
References
Yang L et al. (2012) Aristolochic acid nephropathy: variation in presentation and prognosis. Nephrol Dial Transplant 27:292–298
Anandagoda N et al. (2015) Preventing aristolochic acid nephropathy. Clin J Am Soc Nephrol 10:167–168
Debelle FD et al. (2008) Aristolochic acid nephropathy: a worldwide problem. Kidney Int 74:158–169
Vanherweghem LJ et al. (1998) Misuse of herbal remedies: the case of an outbreak of terminal renal failure in Belgium (Chinese herbs nephropathy). J Altern Complement Med 4:9–13
Baudoux TE et al. (2012) Probenecid prevents acute tubular necrosis in a mouse model of aristolochic acid nephropathy. Kidney Int 82:1105–1113
Sun D et al. (2006) Role of peritubular capillary loss and hypoxia in progressive tubulointerstitial fibrosis in a rat model of aristolochic acid nephropathy. Am J Nephrol 26:363–371
Pozdzik AA et al. (2008) Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int 73:595–607
Yang L et al. (2007) Possible mechanisms explaining the tendency towards interstitial fibrosis in aristolochic acid-induced acute tubular necrosis. Nephrol Dial Transplant 22:445–456
Debelle FD et al. (2002) Aristolochic acids induce chronic renal failure with interstitial fibrosis in salt-depleted rats. J Am Soc Nephrol 13:431–436
Gao R et al. (2000) Aristolochic acid I-induced apoptosis in LLC-PK1 cells and amelioration of the apoptotic damage by calcium antagonist. Chin Med J (Engl) 113:418–424
Romanov V et al. (2011) Glutamate dehydrogenase requirement for apoptosis induced by aristolochic acid in renal tubular epithelial cells. Apoptosis 16:1217–1228
Bunel V et al. (2015) In vitro effects of Panax ginseng in aristolochic acid-mediated renal tubulotoxicity: apoptosis versus regeneration. Planta Med 81:363–372
Dickman KG et al. (2011) Physiological and molecular characterization of aristolochic acid transport by the kidney. J Pharmacol Exp Ther 338:588–597
Zhou L et al. (2010) Activation of p53 promotes renal injury in acute aristolochic acid nephropathy. J Am Soc Nephrol 21:31–41
Luciano RL et al. (2015) Aristolochic acid nephropathy: epidemiology, clinical presentation, and treatment. Drug Saf 38:55–64
Gu HP et al. (2012) Up-regulating relaxin expression by G-quadruplex interactive ligand to achieve antifibrotic action. Endocrinology 153:3692–3700
Cernaro V et al. (2014) Relaxin: new pathophysiological aspects and pharmacological perspectives for an old protein. Med Res Rev 34:77–105
Yoshida T et al. (2013) Relaxin protects against renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 305:F1169–F1176
Moore XL et al. (2007) Relaxin antagonizes hypertrophy and apoptosis in neonatal rat cardiomyocytes. Endocrinology 148:1582–1589
Johnson A et al. (2013) Apoptosis and angiogenesis: an evolving mechanism for fibrosis. FASEB J 27:3893–3901
Liu MC et al. (2003) The nephrotoxicity of Aristolochia manshuriensis in rats is attributable to its aristolochic acids. Clin Exp Nephrol 7:186–194
Li J et al. (2010) Toxicities of aristolochic acid I and aristololactam I in cultured renal epithelial cells. Toxicol In Vitro 24:1092–1097
Raisova M et al. (2001) The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol 117:333–340
Hsu SY et al. (2000) Tissue-specific Bcl-2 protein partners in apoptosis: an ovarian paradigm. Physiol Rev 80:593–614
Linkermann A et al. (2014) Regulated cell death in AKI. J Am Soc Nephrol 25:2689–2701
Lu Z, Xu S (2006) ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58:621–631
Chow BS et al. (2014) Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int 86:75–85
McGuane JT et al. (2011) Relaxin induces rapid dilation of rodent small renal and human subcutaneous arteries via PI3 kinase and nitric oxide. Endocrinology 152:2786–2796
Yoshida T et al. (2014) Protective effects of relaxin against cisplatin-induced nephrotoxicity in rats. Nephron Exp Nephrol 128:9–20
McCullough PA et al. (2015) Novel markers and therapies for patients with acute heart failure and renal dysfunction. Am J Med 128:311–312
Kuwana H et al. (2008) The phosphoinositide-3 kinase gamma-Akt pathway mediates renal tubular injury in cisplatin nephrotoxicity. Kidney Int 73:430–445
Manning BD et al. (2007) AKT/PKB signaling: navigating downstream. Cell 129:1261–1274
Koyasu S et al. (2003) The role of PI3K in immune cells. Nat Immunol 4:313–319
Lan A et al. (2015) Potential role of Akt signaling in chronic kidney disease. Nephrol Dial Transplant 30:385–394
Zhou Y et al. (2009) PI3K/AKT mediated p53 down-regulation participates in CpG DNA inhibition of spontaneous B cell apoptosis. Cell Mol Immunol 6:175–180
Xie L et al. (2006) Role of PI3-kinase/Akt signalling pathway in renal function and cell proliferation after renal ischaemia/reperfusion injury in mice. Nephrology (Carlton) 11:207–212
Acknowledgements
This study is supported by Grants from the Hangzhou Science and Technology Committee (20150733Q06).
Authors contributions
X.C.C and F.X. designed the research protocols and drafted the paper. Z.N., Y.X., and X.Q.H conducted research. C.Q., W.K.X. and M.W. analyzed data and performed statistical analysis. All authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Xie, XC., Zhao, N., Xu, QH. et al. Relaxin attenuates aristolochic acid induced human tubular epithelial cell apoptosis in vitro by activation of the PI3K/Akt signaling pathway. Apoptosis 22, 769–776 (2017). https://doi.org/10.1007/s10495-017-1369-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1369-z