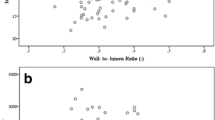
Abstract
Patients with chronic kidney disease (CKD) have an increased risk for cardiovascular morbidity and mortality. Capillary rarefaction may be both one of the causes as well as a consequence of CKD and cardiovascular disease. We reviewed the published literature on human biopsy studies and conclude that renal capillary rarefaction occurs independently of the cause of renal function decline. Moreover, glomerular hypertrophy may be an early sign of generalized endothelial dysfunction, while peritubular capillary loss occurs in advanced renal disease. Recent studies with non-invasive measurements show that capillary rarefaction is detected systemically (e.g., in the skin) in individuals with albuminuria, as sign of early CKD and/or generalized endothelial dysfunction. Decreased capillary density is found in omental fat, muscle and heart biopsies of patients with advanced CKD as well as in skin, fat, muscle, brain and heart biopsies of individuals with cardiovascular risk factors. No biopsy studies have yet been performed on capillary rarefaction in individuals with early CKD. At present it is unknown whether individuals with CKD and cardiovascular disease merely share the same risk factors for capillary rarefaction, or whether there is a causal relationship between rarefaction in renal and systemic capillaries. Further studies on renal and systemic capillary rarefaction, including their temporal relationship and underlying mechanisms are needed. This review stresses the importance of preserving and maintaining capillary integrity and homeostasis in the prevention and management of renal and cardiovascular disease.



Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed for this review article.
References
Levin A, Tonelli M, Bonventre J et al (2017) Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research and policy. Lancet 290:1888–1916. https://doi.org/10.1016/S0140-6736(17)30788-2
Webster AC, Nagler EV, Morton RJ et al (2017) Chronic kidney disease. Lancet 389:1238–1252. https://doi.org/10.1016/S0140-6736(16)32064-5
Stevens PE, Levin A (2013) Evlauation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158:825–830. https://doi.org/10.7326/0003-4819-158-11-201306040-00007
Chade AR (2013) Renal vascular structure and rarefaction. Compr Physiol 3:817–831. https://doi.org/10.1002/cphy.c120012
Ehling J, Babickova J, Gremse F et al (2016) Quantitative micro-computed tomogrphay imaging of vascular dysfunction in progressive kidney diseases. J Am Soc Nephrol 27:520–532. https://doi.org/10.1681/ASN.2015020204
Levy BI, Schiffrin EL, Mourad JJ et al (2008) Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 118:968–976. https://doi.org/10.1161/CIRCULATIONAHA.107.763730
Houben AJHM, Martens RJH, Stehouwer CDA (2017) Assessing microvascular function in humans from a chronic disease perspective. J Am Soc Nephrol 28:3461–3472. https://doi.org/10.1681/ASN.2017020157
Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9:653–660. https://doi.org/10.1038/nm06030653
Eltzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364:656–665. https://doi.org/10.1056/NEJMra0910283
Kang DH, Kanellis J, Hugo C et al (2002) Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13:806–816
Kriz W, LeHir M (2005) Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67:404–419. https://doi.org/10.1111/j.1523-1755.2005.67097.x
Keller G, Zimmer G, Mall G et al (2003) Nephron number in patients with primary hypertension. N Engl J Med 348:101–108. https://doi.org/10.1056/NEJMoa020549
Hughson MD, Douglas-Denton R, Bertram JF et al (2006) Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int 69:671–678. https://doi.org/10.1038/sj.ki.4000041
Hoy WE, Hughson MD, Singh GR et al (2006) Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int 70:104–110. https://doi.org/10.1038/sj.ki.50000397
Denic A, Mathew J, Nagineni VV et al (2018) Clinical and pathology findings associate consistently with larger glomerular volume. J Am Soc Nephrol 29:1960–1969
Lenihan CR, Busque S, Derby G et al (2015) The association of predonation hypertension with glomerular function and number in older living kidney donors. J Am Soc Neprhol 26:1261–1267. https://doi.org/10.11681/ASN.2014030304
Kanzaki G, Puelles VG, Cullen-McEwen LA et al (2017) New insights on glomerular hyperfiltration: a Japanese autopsy study. JCI Insight 5:e94334. https://doi.org/10.1172/jci.insight.94334
Manalich R, Reyes L, Herrera M et al (2000) Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorhometric study. Kidney Int 58:770–773. https://doi.org/10.1046/j.1523-1755.2000.00225.x
Abdi R, Slakey D, Kittur D et al (1998) Baseline glomerular size as a predictor of funcction in human renal transplantation. Transplantation 15:329–333. https://doi.org/10.1097/00007890-1998081150-00009
McNamara BJ, Diouf B, Douglas-Denton RN et al (2010) A comparison of nephron number, glomerular volume and kidney weight in Senegalese Africans and African Americans. Nephrol Dial Transplant 25:1514–1520. https://doi.org/10.1093/ndt/gfq030
Merzhani MA, Denic A, Narasimhan R et al (2021) Kidney microstructural features at time of donation predict long-term risk of chronic kidney disease in living kidney donors. Mayo Clin Proc 96:40–51. https://doi.org/10.1016/j.mayocp.2020.08.041
Denic A, Elsherbiny H, Mulaan AF et al (2020) Larger nephron size and nephrosclerosis predict progressive CKD and mortality after radical nephrectomy for tumor and independent of kidney function. J Am Soc Nephrol 31:2642–2652. https://doi.org/10.1681/ASN.2020040449
Hill GS, Heudes D, Jaquot C et al (2006) Morphometric evidence for impairment of renal autoregulation in advanced essential hypertension. Kidney Int 69:823–831
Hill GS, Heudes D, Barietty J (2003) Morphometric study of arterioles and glomeruli in the aging kidney suggests focal loss of autoregulation. Kidney Int 63:1027–1036. https://doi.org/10.1046/j.1523-1755.2003.99831.x
Hodgin JB, Bizer M, Wickman L et al (2015) Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J Am Soc Nephrol 26:3162–3178. https://doi.org/10.1681/ASN.2014080752
Kriz W, Lemley KV (2015) A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol 26:258–269. https://doi.org/10.1152/ajprenal.00478.2012
Koike K, Ikezumi Y, Tsuboi N et al (2017) Glomerular density and volume in renal biopsy specimens of children with proteinuria relative to preterm birth and gestational age. Clin J Am Soc Nephrol 12:585–590. https://doi.org/10.2215/CJN.05650516
Baelde HJ, Eikmans M, Lappin DW et al (2007) Reduction of VEGF-A and CTGF expression in diabetic nephropathy is associated with podocyte loss. Kidney Int 71:637–645. https://doi.org/10.1038/sj.ki.5002101
Asada N, Tsukahara T, Furuhata M et al (2017) Polycythemia, capillary rarefaction, and focal glomerulosclerosis in two adolescents born extremely low birth weight and premature. Pediatr Nephrol 32:1275–1278. https://doi.org/10.1007/s00467-017-3654-z
Kaplan C, Pasternack B, Shah H et al (1975) Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol 80:227–234
Puelles VG, Douglas-Denton RN, Cullen-McEwen LA et al (2015) Podocyte number in children and adults: association with glomerular size and numbers of other glomerular resident cells. J Am Soc Nephrol 26:2277–2288. https://doi.org/10.1681/ASN.2014070641
Zhu X, Wu S, Dahut WL et al (2007) Risk of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systemic review and meta-analysis. Am J Kidney Dis 49:186–193. https://doi.org/10.1053/j.ajkd.2006.11.039
Eremina V, Jefferson JA, Kowalewska J et al (2008) VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358:1129–1136. https://doi.org/10.1056/NEJMoa0707330
Ellis EN, Steffes MW, Goetz FC et al (1986) Glomerular filtration surface in type 1 diabetes mellitus. Kidney Int 29:889–894. https://doi.org/10.1038/ki.1986.82
Navar LG, Maddox DA, Munger KA (2020) The renal circulations and glomerular filtration. In Yu ASL, Chertow GM, Luyckx VA et al. (Eds.) Brenner and rector’s the kidney, 11th Ed, pp 80–114
Evans RG, Ince C, Joles JA et al (2013) Haemodynamic influences on kidney oxygenation: clinical implications of integrative physiology. Clin Exp Pharmacol Physiol 40:106–122. https://doi.org/10.1111/1440-1681.12031
Farris AB, Ellis CL, Rogers TE et al (2016) Renal medullary and cortical correlates in fibrosis, epithelial mass, microvascularity, and microanatomy using whole slide image analysis morphometry. PLoS ONE 11:e0161019. https://doi.org/10.1371/journal.pone.0161019
Konda R, Sato H, Sakai K et al (1999) Expression of platelet-derived endothelial cell growth factor and its potential role in up-regulation of angiogenesis in scarred kidneys secondary to urinary tract diseases. Am J Pathol 155:1587–1597. https://doi.org/10.1016/S0002-9440(10)65475-2
Choi YJ, Chakraborty S, Nguyen V et al (2000) Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum Pathol 31:1491–1497. https://doi.org/10.1053/hupa.2000.20373
Namikoshi T, Satoh M, Horike H et al (2006) Implication of peritubular capillary loss and altered expression of vascular endothelial growth factor in IgA nephropathy. Nephron Physiol 102:9–16. https://doi.org/10.1159/000088405
Ozdemir BH, Demirhan B, Ozdemir FN et al (2004) The role of microvascular injury on steroid and OKT3 response in renal allograft rejection. Transplantation 78:734–740. https://doi.org/10.1097/01.tp.0000130453.79906.62
Anutrakulchai S, Titipungul T, Pattay T et al (2016) Relation of peritubular capillary features to class of lupus nephritis. BMC Nephrol 17:169. https://doi.org/10.1186/s12882-016-0388-2
Seron D, Alexopoulos E, Raftery MJ et al (1990) Number of interstitial capillary cross-sections assessed by monoclonal antibodies: relation to interstitial damage. Nephrol Dial Transplant 5:889–893. https://doi.org/10.1093/ndt/5.10.889
Bohle A, Mackensen-Haen S, Wehrmann M (1996) Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res 19:191–195. https://doi.org/10.1159/00017072
Ishii Y, Sawada T, Kubota K et al (2005) Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int 67:321–332. https://doi.org/10.1111/j.1523-1755.2005.00085.x
Lindenmeyer MT, Kretzler M, Boucherot A et al (2007) Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol 18:1765–1776. https://doi.org/10.1681/ASN.2006121304
Klomjit N, Zhu XY, Eirin A et al (2022) Mirovascular remodeling and altered angiogenic singaling in human kidneys distal to occlusive atherosclerotic renal artery stenosis. Nephrol Dial Transplant 37:1844–1856. https://doi.org/10.1093/ndt/gfac156
Steegh FM, Gelens MA, Nieman FH et al (2011) Early loss of peritubular capillaries after kidney transplantation. J Am Soc Nephrol 22:1024–1029. https://doi.org/10.1681/ASN.2010050531
Sutton TA, Fisher CJ, Molitoris BA (2002) Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62:1539–1549. https://doi.org/10.1046/j.1523-1755.2002.00631.x
Babickova J, Klinkhammer BM, Buhl EM et al (2017) Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int 91:70–85. https://doi.org/10.1016/j.kint.2016.07.038
Kida Y, Duffield JS (2011) Pivotal role of pericytes in kidney fibrosis. Clin Exp Pharmacol Phyisol 38:467–473. https://doi.org/10.1111/j.1440-1681.2011.0531.x
Grgic I, Campanholle G, Bijol V et al (2012) Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82:172–183. https://doi.org/10.1038/ki.2012.20
Kramann R, Wongboonsin J, Chang-Panesso M et al (2017) Gli1+ pericyte loss induces capillary rarefaction and proximal tubular injury. J Am Soc Nephrol 28:776–784. https://doi.org/10.1681/ASN.2016030297
Humphreys BD, Lin SL, Kobayashi A et al (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblats in kidney fibrosis. Am J Pathol 176:85–97. https://doi.org/10.2353/ajpath.2010.090517
Dimke H, Sparks MA, Thomson BR et al (2015) Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in the kidney. J Am Soc Nephrol 26:1027–1038. https://doi.org/10.1681/ASN.201410060
Querfeld U, Mark RH, Pries AR (2020) Microvascular disease in chronic kidney disease: the base of the iceberg in cardiovascular comorbidity. Clin Sci (Lond) 134:1333–1356. https://doi.org/10.1042/CS20200279
Charytan DM, Padera R, Helfand AM et al (2014) Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol 176:99–109. https://doi.org/10.1016/j.ijcard.2014.06.062
Bongard O, Weimer D, Lemoine R et al (2000) Cyclosporine toxicity in renal transplant recipients detected by nailfold capillaroscopy with Na-fluorescein. Kidney Int 58:2559–2563. https://doi.org/10.1046/j.1523-1755.2000.00441.x
Bijkerk R, Florijn BW, Khairoun M et al (2017) Acute rejection after kidney transplantation associates with circulating microRNAs and vascular injury. Transplant Direct 19:e174. https://doi.org/10.1097/TXD.0000000000000699
Martens RJ, Henry RM, Houben AJ et al (2016) Capillary rarefaction associates with albuminuria: the Maastricht study. J Am Soc Nephrol 27:3748–3757. https://doi.org/10.1681/ASN.2015111219
Triantafyllou A, Anyfanti P, Zabulis X et al (2014) Accumulation of microvascular target organ damage in newly diagnosed hypertensive patients. J Am Soc Hypertens 8:542–549. https://doi.org/10.1016/j.jash.2014.04.008
Martens RJH, Houben AJHM, Kooman JP et al (2018) Microvascular endothelial dysfunction is associated with albuminuria: the Maastricht Study. J Hypertens 36:1178–1187. https://doi.org/10.1097/HJH.00000000000001674
Matsushita K, van der Velde M, Astor BC et al (2010) Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375:2073–2081. https://doi.org/10.1016/S0140-6736(10)60674-5
van Hecke MV, Dekker JM, Nijpels G et al (2005) Inflammation and endothelial dysfunction are associated with retinopathy: the Hoorn study. Diabetologia 48:1300–1306. https://doi.org/10.1007/s00125-005-1799-y
Gerstein HC, Mann JF, Yi Q et al (2001) Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286:421–426. https://doi.org/10.1001/jama.286.4.421
Martens RJ, Kooman JP, Stehouwer CD et al (2017) Estimated GFR, albuminuria, and cognitive performance: the Maastricht study. Am J Kidney Dis 69:179–191. https://doi.org/10.1053/j.ajkd/2016.04.017
Martens RJ, Kooman JP, Stehouwer CDA et al (2018) Albuminuria is associated with a higher prevalence of depression in a population-based cohort study: the Maastricht Study. Nephrol Dial Transplant 33:128–138. https://doi.org/10.1093/ndt/gfw377
Li W, Schram MT, Soerensen BM et al (2020) Microvascular phenotyping in the Maastricht study: design and main findings 2010–2018. Am J Epidemiol 189:873–884. https://doi.org/10.1093/aje/kwaa023
Fioretto P, Stehouwer CD, Mauer M et al (1998) Heterogeneous nature of microalbuminuria in NIDDM: studies of endothelial function and renal structure. Diabetologia 41:233–236. https://doi.org/10.1007/s001250050895
Paiardi S, Rodella LF, De Ciuceis C et al (2009) Immunohistochemical evaluation of microvascular rarefaction in hypertensive humans and in spontaneously hypertensive rats. Clin Hemorheol Microcirc 42:259–268. https://doi.org/10.3233/CH-2009-1195
Marin P, Andersson B, Krotkiewski M et al (1994) Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 17:382–386. https://doi.org/10.2337/diacare.17.5.382
Hernandez N, Torres SH, Finol HJ et al (1999) Capillary changes in skeletal muscle of patients with essential hypertension. Anat Rec 256:425–432. https://doi.org/10.1002/(SICI)1097-0185(19991201)256:4
Gueugneau M, Coudy-Gandilhon C, Meunier B et al (2016) Lower skeletal muscle capillarization in hypertensive elderly men. Exp Gerontol 76:80–88. https://doi.org/10.1016/j.exger.2016.01.013
Pasarica M, Sereda OR, Redman LM et al (2009) Reduced adipose tissue oxygenation in human obesity; evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58:718–725. https://doi.org/10.2337/db08-1098
Pasarica M, Rood J, Ravussin E et al (2010) Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab 95:4052–4055. https://doi.org/10.1210/jc.2009-2377
Spencer M, Unal R, Zhu B et al (2011) Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab 96:e1990-1998. https://doi.org/10.1210/jc2011-1567
Goossens GH, Bizzarri A, Venteclef N et al (2011) Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 124:67–76. https://doi.org/10.1161/CIRCULATIONAHA.111.027813
Belligoli A, Compagnin C, Sanna M et al (2019) Characterization of subcutaneous and omental adipose tissue in patients with obesity and with different degrees of glucose impairment. Sci Rep 9:11333. https://doi.org/10.1038/s41598-019-47719-y
Chilibeck PD, Paterson DH, Cunningham DA et al (1985) Muscle capillarization O2 diffustion distance, and VO2 kinetics in old and young individuals. J Appl Physiol 1997(82):63–69. https://doi.org/10.1152/jappl.1997.82.1.63
De Ciuceis C, Cornali C, Porteri E et al (2014) Cerebral small-resistance artery structure and cerebral blood flow in normotensive subjects and hypertensive patients. Neuroradiology 56:1103–1111. https://doi.org/10.1007/s00234-014-1423-2
Amann K, Breitbach M, Ritz E et al (1998) Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol 9:1018–1022. https://doi.org/10.1681/ASN.V961018
Yarom R, Sack S, Sapoznikov D et al (1994) Myocardial capillaries and autonomic nerves in diabetes: morphometric study of auricles from bypass surgery biopsies. Cardiovasc Pathol 3:43–50. https://doi.org/10.1016/1054-8807(94)90006-X
Campbell DJ, Somaratne JB, Jenkins AJ et al (2011) Differences in myocardial structure and coronary microvasculature between men and women with coronary artery disease. Cardiovasc Diabetol 57:186–192. https://doi.org/10.1161/HYPERTENSIONAHA.110.165043
Campbell DJ, Somaratne JB, Prior DL et al (2013) Obesity is associated with lower coronary microvascular density. PLoS ONE 8:e81798. https://doi.org/10.1371/journal.pone.0081798
Hinkel R, Howe A, Renner S et al (2017) Diabetes mellitus-induced microvascular destabilization in the myocardium. J Am Coll Cardiol 17(69):131–143. https://doi.org/10.1016/j.jacc.2016.10.058
Kawaguchi M, Techigawara M, Ishihata T et al (1997) A comparison of ultrastructural changes on endoymocardial biopsy specimens obtained from patients with diabetes mellitus with and without hypertension. Heart Vessels 12:267–274. https://doi.org/10.1007/BF02766802
Yarom R, Zirkin H, Staemmler G et al (1992) Human coronary microvessles in diabetes and ischaemia: Morphometric study of autopsy material. J Pathol 166:265–270. https://doi.org/10.1002/path.1711660308
Brown WR, Thore CR (2011) Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol 37:56–74. https://doi.org/10.1111/j.1365-2990.2010.01139.x
Hunter JM, Kwan J, Malek-Ahmadi M et al (2012) Morphological and pathological evaluation of the brain microcirculation in aging and Alzheimer’s disease. PLoS ONE 7:e36893. https://doi.org/10.1371/journal.pone.0036893
Kalari RN, Hedera P (1995) Differentital degeneration of the cerebral microvasculature in Alzheimer’s disease. NeuroReport 15:477–480. https://doi.org/10.1097/00001756-199502000-00018
Szpak GM, Lewandowska E, Wierzba-Bobrowicz T et al (2007) Small cerebral vessel disease in familial amyolid and non-amyloid angiopathies: FAD-PS-1 (P117L) mutation and CADASIL Immunohistochemical and ultrastructural studies. Folia Neuropathol 45:192–204
Livingston G, Huntley J, Sommerlad A et al (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet 396:413–446. https://doi.org/10.1016/S0140-6736(20)30367-6
Scheffer S, Hermkens DMA, van der Weerd L et al (2021) Vascular hypothesis of Alzheimer disease: topical review of mouse models. Aterioscler Thromb Vasc Biol 41:1265–1283. https://doi.org/10.1161/ATVBAHA.120.311911
Groen BB, Hamer HM, Snijders T et al (1985) Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol 2014(116):998–1005. https://doi.org/10.1152/japplphysiol.009.2013
Ellis EN, Mauer SM, Goetz FC et al (1986) Relationship of muscle capillary basement membrane to renal structure and function in diabetes mellitus. Diabetes 35:421–425. https://doi.org/10.2337/diab.35.4.421
Rossitto G, Mary S, McAllister C et al (2020) Reduced lymphatic reserve in heart failure with preserved ejection fraction. J Am Coll Cardiol 76:2817–2829. https://doi.org/10.1016/j.jacc.2020.10.022
Anderson M, Forrest Parrott C, Haykowsky MJ et al (2022) Skeletal muscle abnormalities in heart failure with preserved ejection fraction. Heart Fail Rev. https://doi.org/10.1007/s10741-10219
Mohammed SF, Hussain S, Mirzoyev SA et al (2015) Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 131:550–559. https://doi.org/10.1161/CIRCULATIONAHA.114.009625
Broyd CJ, Hernandez-Perez F, Segovia J et al (2018) Identification of capillary rarefaction using intracoronary wave intensity analysis with resultant prognostic implications for cardiac allograft patients. Eur Heart J 39:1807–1814. https://doi.org/10.1093/eurheartj/ehx732
Wadowski PP, Huelsmann M, Schoergenhofer C et al (2018) Sublingual functional capillary rarefaction in chronic heart failure. Eur J Clin Invest. https://doi.org/10.1111/eci.12869
Houben AJ, Beljaars JH, Hofstra L et al (2003) Microvascular abnormalities in chronic heart failure: a cross-sectional analysis. Microcirculation 10:471–478. https://doi.org/10.1038/sj.mn.7800211
Garg AX, Nevis IF, McArthur E et al (2015) Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med 372:124–133. https://doi.org/10.1056/NEJMoa1408932
Lane BR, Demirjian S, Derweesh IH et al (2015) Survival and functional stability in chronic kidney disease due to surgical removal of nephrons: importance of the new baseline glomerular filtration rate. Eur Urol 68:996–1003. https://doi.org/10.1016/j.eururo.2015.04.043
Schoina M, Loutradis C, Memmos E et al (2021) Microcirculatory function deteriorates with advancing stages of chronic kidney disease independently of arterial stiffness and atherosclerosis. Hypertens Res 44:179–187
Baumann M, Burkhardt K, Heemann U (2014) Microcirculatory marker for the prediction of renal end points: a prospective cohort study in patients with chronic kidney disease stage 2–4. Hypertension 64:338–346. https://doi.org/10.1161/HYPERTENSIONAHA.114.03354
Yau JW, Xie J, Kawasaki R et al (2011) Retinal arteriolar narrowing and subsequent development of CKD stage3: the multi-ethnic study of atherosclerosis (MESA). Am J Kidney Dis 58:39–46. https://doi.org/10.1053/j.ajkd.2011.02.382
Sabanayagam C, Shankar A, Klein BE et al (2011) Biderectional association of retinal vessel diameters and estimated GFR decline: the Beaver DAM CKD study. Am J Kidney Dis 57:682–691. https://doi.org/10.1053/j.ajkd.2010.11.025
Yip W, Ong PG, Teo BW et al (2017) Retinal vascular imaging markers and incident chronic kidney disease: a prospective cohort study. Sci Rep 7:9374. https://doi.org/10.1038/s41598-017-09204-2
Johnson RJ, Herrera-Acosta J, Schreiner GF et al (2002) Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Eng J Med 346:913–923. https://doi.org/10.1056/NEJMra011078
Johnson RJ, Schreiner GF (1997) Hypothesis: the role of acquired tubulo–interstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int 52:1169–1179. https://doi.org/10.1038/ki/1997.442
Libby P, Luescher T (2020) Covid-19 is, in the end, an endothelial disease. Eur Heart J 41:3038–3044. https://doi.org/10.1093/eurheartj/ehaa623
Osiaevi I, Schulze A, Evers G, Harmening K, Vink H, Kuempers P, Mohr M, Rovas A (2023) Persistent capillary rarefication in long COVID syndrome. Angiogenesis 26:53–61. https://doi.org/10.1007/s10456-022-09850-9
Katz A, Caramori ML, Sisson-Ross S et al (2002) An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int 61:2058–2066. https://doi.org/10.1046/j.1523-1755.2002.00370.x
Yang L, Li X, Wang H (2007) Possible mechanisms explaining the tendency towards interstitial fibrosis in aristolochic acid-induced acute tubular necrosis. Nephrol Dial Transplant 22:445–456. https://doi.org/10.1093/ndt/gfl556
Kaukinen A, Lautenschlager I, Helin H et al (2009) Peritubular capillaries are rarefied in congenital nephrotic syndrome of the Finnish type. Kidney Int 75:1099–1108. https://doi.org/10.1038/ki/2009.41
Thacker SG, Berthier CC, Mattinzoli D et al (2010) The detrimental effects of IFN-alpha on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J Immunol 185:4457–4469. https://doi.org/10.4049/jimmunol.1001782
Kimura N, Kimura H, Takahashi N et al (2015) Renal resistance index correlates with peritubular capillary loss and arteriosclerosis in biopsy tissues from patients with chronic kidney disease. Clin Exp Nephrol 19:1114–1119. https://doi.org/10.1007/s10157-015-1116-0
von Stillfried S, Apitzsch JC, Ehling J et al (2016) Contrast-enhanced CT imaging in patients with chronic kidney disease. Angiogenesis 19:525–535. https://doi.org/10.1007/s10456-016-9524-7
Sun IO, Santelli A, Abumoawad A et al (2018) Loss of peritubular capillaries in hypertensive patients is detectable by urinary endothelial microparticle levels. Hypertension 72:1180–1188. https://doi.org/10.1161/HYPERTENSIONAHA.118.11766
Henrich HA, Romen W, Heimgaertner W et al (1988) Capillary rarefaction characteristic of het skeletal muscle of hypertensive patients. Klin Wochenschr 66:54–60. https://doi.org/10.1007/BF01713011
Gavin TP, Stallings HW, Zwetsloot KA et al (1985) Lower capillary density but no difference in VEGF expression in obese vs lean young skeletal muscle in humans. J Appl Physiol 2005(98):315–321. https://doi.org/10.1152/japplphysiol.00353.2004
Croley AN, Zwetsloot KA, Westerkamp LM et al (1985) Lower capillarization, VEGF protein, and VEGF mRNA response to acute exercise in the vastus lateralis muscle of aged vs young women. J Appl Physiol 2005(99):1872–1879. https://doi.org/10.1152/japplphysiol.00498.2005
Ryan NA, Zwetsloot KA, Westerkap LM et al (1985) Lower skeletal muscle capillarization and VEGF expression in aged vs young men. J Appl Phyisol 2006(100):178–185. https://doi.org/10.1152/japplphysiol.00827.2005
Prior SJ, Goldberg AP, Ortmeyer HK et al (2015) Increased skeletal muscle capillarization independently enhances insulin sensitivity in older adults after exercise training and detraining. Diabetes 64:3386–3395. https://doi.org/10.2337/db14-1771
Rizzoni D, De Ciuceis C, Porteri E et al (2009) Altered structure of small cerebral arteries in patients with essential hypertension. J Hypertens 27:838–845. https://doi.org/10.1097/HJH.0b013e32832401ea
Funding
This work was partly financially supported by research grants from the Dutch Kidney Foundation (Grant DKF 13A1D303) and the “Profileringsfonds” of the Academic Hospital Maastricht (Grant PF292). Gert Jan Zonneveld (Audiovisual support of Maastricht UMC +) is thanked for design of Fig. 1.
Author information
Authors and Affiliations
Contributions
FS, MD and CPK developed the concepts described in this review. AK, PH, TR, AH, KR, and CS further contributed to the concepts. FS and CPK performed literature research. All authors were involved in writing the paper and gave their final approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Steegh, F.M.E.G., Keijbeck, A.A., de Hoogt, P.A. et al. Capillary rarefaction: a missing link in renal and cardiovascular disease?. Angiogenesis 27, 23–35 (2024). https://doi.org/10.1007/s10456-023-09883-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-023-09883-8