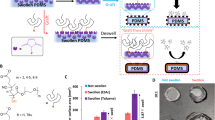
Abstract
Device failure due to undesired biological responses remains a substantial roadblock in the development and translation of new devices into clinical care. Polyethylene glycol (PEG)-based hydrogel coatings can be used to confer antifouling properties to medical devices—enabling minimization of biological responses such as bacterial infection, thrombosis, and foreign body reactions. Application of hydrogel coatings to diverse substrates requires careful consideration of multiple material factors. Herein, we report a systematic investigation of two coating methods: (1) traditional photoinitiated hydrogel coatings; (2) diffusion-mediated, redox-initiated hydrogel coatings. The effects of method, substrate, and compositional variables on the resulting hydrogel coating thickness are presented. To expand the redox-based method to include high molecular weight macromers, a mechanistic investigation of the role of cure rate and macromer viscosity was necessary to balance solution infiltration and gelation. Overall, these structure–property relationships provide users with a toolbox for hydrogel coating design for a broad range of medical devices.






Similar content being viewed by others
References
Anseth, K. S., K. J. Anderson, and C. N. Bowman. Radical concentrations, environments, and reactivities during crosslinking polymerizations. Macromol. Chem. Phys. 197:833–848, 1996.
Anseth, K. S., and C. N. Bowman. Kinetic gelation model predictions of crosslinked polymer network microstructure. Chem. Eng. Sci. 49:2207–2217, 1994.
Anseth, K. S., L. M. Kline, T. A. Walker, K. J. Anderson, and C. N. Bowman. Reaction kinetics and volume relaxation during polymerizations of multiethylene glycol dimethacrylates. Macromolecules. 28:2491–2499, 1995.
Ariff, M., M. Jainuddin, V. Gopalan, and K. V. Rao. Aqueous polymerization of acrylonitrile by ascorbic acid–peroxodisulfate redox system. J. Polym. Sci. Polym. Chem. Ed. 23:2063–2071, 1985.
Berry, K. L., and J. H. Peterson. Tracer studies of oxidation—Reduction polymerization and molecular weight of “Teflon” tetrafluoroethylene resin. J. Am. Chem. Soc. 73:5195–5197, 1951.
Bowman, C. N., and N. A. Peppas. Coupling of kinetics and volume relaxation during polymerizations of multiacrylates and multimethacrylates. Macromolecules. 24:1914–1920, 1991.
Bowman, C. N., and N. A. Peppas. A kinetic gelation method for the simulation of free-radical polymerizations. Chem. Eng. Sci. 47:1411–1419, 1992.
Browning, M. B., D. Dempsey, V. Guiza, S. Becerra, J. Rivera, et al. Multilayer vascular grafts based on collagen-mimetic proteins. Acta Biomater. 8:1010–1021, 2012.
Browning, M. B., V. Guiza, B. Russell, J. Rivera, S. Cereceres, et al. Endothelial cell response to chemical, biological, and physical cues in bioactive hydrogels. Tissue Eng. Part A. 20:3130–3141, 2014.
Browning, M. B., B. Russell, J. Rivera, M. Höök, and E. M. Cosgriff-Hernandez. Bioactive Hydrogels with enhanced initial and sustained cell interactions. Biomacromolecules. 14:2225–2233, 2013.
Browning, M., T. Wilems, M. Hahn, and E. Cosgriff-Hernandez. Compositional control of poly (ethylene glycol) hydrogel modulus independent of mesh size. J. Biomed. Mater. Res. Part A. 98:268–273, 2011.
Chang, J., Y. Tao, B. Wang, X. T. Yang, H. Xu, et al. Evaluation of a redox-initiated in situ hydrogel as vitreous substitute. Polymer. 55:4627–4633, 2014.
Chapla, R., M. Alhaj Abed, and J. West. Modulating functionalized poly(ethylene glycol) diacrylate hydrogel mechanical properties through competitive crosslinking mechanics for soft tissue applications. Polymers. 12:3000, 2020.
Chen, K., Y. Feng, Y. Zhang, L. Yu, X. Hao, et al. Entanglement-driven adhesion, self-healing, and high stretchability of double-network PEG-based hydrogels. ACS Appl. Mater. Interfaces. 11:36458–36468, 2019.
Cosgriff-Hernandez, E., M. S. Hahn, B. Russell, T. Wilems, D. Munoz-Pinto, et al. Bioactive hydrogels based on designer collagens. Acta Biomater. 6:3969–3977, 2010.
Cosgriff-Hernandez, E., A. Post, P. Dhavalikar, T. Wilems, and Z. Lan, Integrin-targeting materials in regenerative medicine, in Proceedings of Abstracts of Papers of the American Chemical Society, vol. 256. Washington, DC: American Chemical Society, 2018.
Dortdivanlioglu, B., N. E. D. Yilmaz, K. B. Goh, X. Zheng, and C. Linder. Swelling-induced interface crease instabilities at hydrogel bilayers. J. Elasticity. 145:31–47, 2021.
Fordham, J. W. L., and H. L. Williams. The persulfate-iron (II) initiator system for free radical polymerizations1. J. Am. Chem. Soc. 73:4855–4859, 1951.
Fu, M., Y. Liang, X. Lv, C. Li, Y. Y. Yang, et al. Recent advances in hydrogel-based anti-infective coatings. J. Mater. Sci. Technol. 85:169–183, 2021.
Fuchs, S., K. Shariati, and M. L. Ma. Specialty tough hydrogels and their biomedical applications. Adv. Healthc. Mater. 9(2):e1901396, 2020.
Gold, G. T., D. M. Varma, P. J. Taub, and S. B. Nicoll. Development of crosslinked methylcellulose hydrogels for soft tissue augmentation using an ammonium persulfate-ascorbic acid redox system. Carbohydr. Polym. 134:497–507, 2015.
House, D. A. Kinetics and mechanism of oxidations by peroxydisulfate. Chem. Rev. 62:185–203, 1962.
Hume, P. S., C. N. Bowman, and K. S. Anseth. Functionalized PEG hydrogels through reactive dip-coating for the formation of immunoactive barriers. Biomaterials. 32:6204–6212, 2011.
Jana, S. Endothelialization of cardiovascular devices. Acta Biomater. 99:53–71, 2019.
Jeitner, T. M. Optimized ferrozine-based assay for dissolved iron. Anal. Biochem. 454:36–37, 2014.
Jiao, Y., D. Gyawali, J. M. Stark, P. Akcora, P. Nair, et al. A rheological study of biodegradable injectable PEGMC/HA composite scaffolds. Soft Matter. 8:1499–1507, 2012.
Johnson, L. M., C. A. DeForest, A. Pendurti, K. S. Anseth, and C. N. Bowman. Formation of three-dimensional hydrogel multilayers using enzyme-mediated redox chain initiation. ACS Appl. Mater. Interfaces. 2:1963–1972, 2010.
Johnson, L. M., B. D. Fairbanks, K. S. Anseth, and C. N. Bowman. Enzyme-mediated redox initiation for hydrogel generation and cellular encapsulation. Biomacromolecules. 10:3114–3121, 2009.
Kishan, A. P., and E. M. Cosgriff-Hernandez. Recent advancements in electrospinning design for tissue engineering applications: a review. J. Biomed. Mater. Res. Part A. 105:2892–2905, 2017.
Kloosterboer, J. G., Network formation by chain crosslinking photopolymerization and its applications in electronics. In: Electronic Applications. Springer, Berlin, 1988, pp. 1–61.
Krsko, P., and M. Libera. Biointeractive hydrogels. Materials Today. 8:36–44, 2005.
Lee, S., X. Tong, and F. Yang. Effects of the poly(ethylene glycol) hydrogel crosslinking mechanism on protein release. Biomater. Sci. 4:405–411, 2016.
Lenka, S., and A. K. Dhal. Polymerization of acrylonitrile initiated by K2S2O8–Fe (II) redox system. J. Polym. Sci. Polym. Chem. Ed. 19:2115–2118, 1981.
Li, J., A. D. Celiz, J. Yang, Q. Yang, I. Wamala, et al. Tough adhesives for diverse wet surfaces. Science. 357:378–381, 2017.
Lin, C.-C., and K. S. Anseth. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 26:631–643, 2009.
Liu, K., F. Zhang, Y. Wei, Q. Hu, Q. Luo, et al. Dressing blood-contacting materials by a stable hydrogel coating with embedded antimicrobial peptides for robust antibacterial and antithrombus properties. ACS Appl. Mater. Interfaces. 13:38947–38958, 2021.
Liu, Y., W. He, Z. Zhang, and B. P. Lee. Recent developments in tough hydrogels for biomedical applications. Gels. 4:46, 2018.
Lutz, J.-F. Polymerization of oligo(ethylene glycol) (meth)acrylates: toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 46:3459–3470, 2008.
Ma, S., C. Yan, M. Cai, J. Yang, X. Wang, et al. Continuous surface polymerization via Fe (II)-mediated redox reaction for thick hydrogel coatings on versatile substrates. Adv. Mater. 30:1803371, 2018.
Motiwale, S., M. D. Russell, O. Conroy, J. Carruth, M. Wancura, et al. Anisotropic elastic behavior of a hydrogel-coated electrospun polyurethane: suitability for heart valve leaflets. J. Mech. Behav. Biomed. Mater. 125:104877, 2021.
Parada, G., Y. Yu, W. Riley, S. Lojovich, D. Tshikudi, et al. Ultrathin and robust hydrogel coatings on cardiovascular medical devices to mitigate thromboembolic and infectious complications. Adv. Healthc. Mater. 9(20):e2001116, 2020.
Pashneh-Tala, S., S. MacNeil, and F. Claeyssens. The tissue-engineered vascular graft—past, present, and future. Tissue Eng. Part B Rev. 22:68–100, 2016.
Post, A., A. P. Kishan, P. Diaz-Rodriguez, E. Tuzun, M. Hahn, and E. Cosgriff-Hernandez. Introduction of sacrificial bonds to hydrogels to increase defect tolerance during suturing of multilayer vascular grafts. Acta Biomater. 69:313–322, 2018.
Puperi, D. S., A. Kishan, Z. E. Punske, Y. Wu, E. Cosgriff-Hernandez, et al. Electrospun polyurethane and hydrogel composite scaffolds as biomechanical mimics for aortic valve tissue engineering. ACS Biomater. Sci. Eng. 2:1546–1558, 2016.
Richbourg, N. R., M. Wancura, A. E. Gilchrist, S. Toubbeh, B. A. C. Harley, et al. Precise control of synthetic hydrogel network structure via linear, independent synthesis-swelling relationships. Sci. Adv. 7:eabe3245, 2021.
Roseen, M. A., M. M. Fahrenholtz, J. P. Connell, and K. J. Grande-Allen. Interfacial coating method for amine-rich surfaces using poly (ethylene glycol) diacrylate applied to bioprosthetic valve tissue models. ACS Appl. Bio Mater. 3:1321–1330, 2020.
Roseen, M. A., R. Lee, A. D. Post, M. Wancura, J. P. Connell, et al. Poly(ethylene glycol)-based coatings for bioprosthetic valve tissues: toward restoration of physiological behavior. ACS Appl. Bio Mater. 3:8352–8360, 2020.
Scranton, A. B., C. N. Bowman, J. Klier, and N. A. Peppas. Polymerization reaction dynamics of ethylene glycol methacrylates and dimethacrylates by calorimetry. Polymer. 33:1683–1689, 1992.
Singh, Y., D. Gao, Z. Gu, S. Li, K. A. Rivera, et al. Influence of molecular size on the retention of polymeric nanocarrier diagnostic agents in breast ducts. Pharm. Res. 29:2377–2388, 2012.
Spencer, K. C., J. C. Sy, K. B. Ramadi, A. M. Graybiel, R. Langer, and M. J. Cima. Characterization of mechanically matched hydrogel coatings to improve the biocompatibility of neural implants. Sci. Rep. 7:1952, 2017.
Stevens, K. R., J. S. Miller, B. L. Blakely, C. S. Chen, and S. N. Bhatia. Degradable hydrogels derived from PEG-diacrylamide for hepatic tissue engineering. J. Biomed. Mater. Res. Part A. 103:3331–3338, 2015.
Stevens, M. M. Biomaterials for bone tissue engineering. Mater. Today. 11:18–25, 2008.
Tatterton, M., S.-P. Wilshaw, E. Ingham, and S. Homer-Vanniasinkam. The use of antithrombotic therapies in reducing synthetic small-diameter vascular graft thrombosis. Vasc. Endovasc. Surg. 46:212–222, 2012.
Temenoff, J. S., H. Shin, D. E. Conway, P. S. Engel, and A. G. Mikos. In vitro cytotoxicity of redox radical initiators for cross-linking of oligo (poly (ethylene glycol) fumarate) macromers. Biomacromolecules. 4:1605–1613, 2003.
Tseng, H., D. S. Puperi, E. J. Kim, S. Ayoub, J. V. Shah, et al. Anisotropic poly(ethylene glycol)/polycaprolactone hydrogel-fiber composites for heart valve tissue engineering. Tissue Eng. Part A. 20:2634–2645, 2014.
Wancura, M., M. Talanker, S. Toubbeh, A. Bryan, and E. Cosgriff-Hernandez. Bioactive hydrogel coatings of complex substrates using diffusion-mediated redox initiation. J. Mater. Chem. B. 8:4289–4298, 2020.
Whitely, M., S. Cereceres, P. Dhavalikar, K. Salhadar, T. Wilems, et al. Improved in situ seeding of 3D printed scaffolds using cell-releasing hydrogels. Biomaterials. 185:194–204, 2018.
Wilems, T. S., X. Lu, Y. E. Kurosu, Z. Khan, H. J. Lim, and L. A. S. Callahan. Effects of free radical initiators on polyethylene glycol dimethacrylate hydrogel properties and biocompatibility. J. Biomed. Mater. Res. Part A. 105:3059–3068, 2017.
Yang, J., R. Bai, B. Chen, and Z. Suo. Hydrogel adhesion: a supramolecular synergy of chemistry, topology, and mechanics. Adv. Funct. Mater. 30(2):1901693, 2019.
Yong, Y., M. Y. Qiao, A. Chiu, S. Fuchs, Q. S. Liu, et al. Conformal hydrogel coatings on catheters to reduce biofouling. Langmuir. 35:1927–1934, 2019.
Yu, Y., H. Yuk, G. A. Parada, Y. Wu, X. Liu, et al. Multifunctional “hydrogel skins” on diverse polymers with arbitrary shapes. Adv. Mater. 31(7):e1807101, 2018.
Yuk, H., T. Zhang, S. Lin, G. A. Parada, and X. Zhao. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 15:190, 2016.
Zhang, F., C. Hu, L. Yang, K. Liu, Y. Ge, et al. A conformally adapted all-in-one hydrogel coating: towards robust hemocompatibility and bactericidal activity. J. Mater. Chem. B. 9:2697–2708, 2021.
Zhang, X., B. Xu, D. S. Puperi, A. L. Yonezawa, Y. Wu, et al. Integrating valve-inspired design features into poly(ethylene glycol) hydrogel scaffolds for heart valve tissue engineering. Acta Biomater. 14:11–21, 2015.
Zhang, Y., D. An, Y. Pardo, A. Chiu, W. Song, et al. High-water-content and resilient PEG-containing hydrogels with low fibrotic response. Acta Biomater. 53:100–108, 2017.
Zhu, J., and R. E. Marchant. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices. 8:607–626, 2011.
Acknowledgments
The work presented here was supported by the National Institutes of Health R21 EB020978. The Bionate® Thermoplastic Polycarbonate-urethane was provided by DSM Biomedical (Berkeley, CA).
Conflict of interest
There are no conflicts to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Emmanuel Opara oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wancura, M., Nkansah, A., Robinson, A. et al. PEG-Based Hydrogel Coatings: Design Tools for Biomedical Applications. Ann Biomed Eng (2023). https://doi.org/10.1007/s10439-023-03154-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-023-03154-9