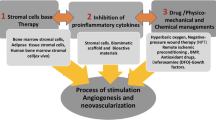
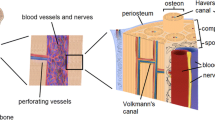
Abstract
The reconstruction of large skeletal defects is still a tricky challenge in orthopedics. The newly formed bone tissue migrates sluggishly from the periphery to the center of the scaffold due to the restrictions of exchange of oxygen and nutrition impotent cells osteogenic differentiation. Angiogenesis plays an important role in bone reconstruction and more and more studies on angiogenesis in bone tissue engineering had been published. Promising advances of angiogenesis in bone tissue engineering by scaffold designs, angiogenic factor delivery, in vivo prevascularization and in vitro prevascularization are discussed in detail. Among all the angiogenesis mode, angiogenic factor delivery is the common methods of angiogenesis in bone tissue engineering and possible research directions in the future.



Similar content being viewed by others
References
Alazraki, N., J. Moitoza, J. Heaphy, and A. Taylor Jr. The effect of iatrogenic trauma on the bone scintigram: an animal study: concise communication. J. Nucl. Med. 25:978–981, 1984.
Alge, D. L., and T. M. G. Chu. Calcium phosphate cement reinforcement by polymer infiltration and in situ curing: a method for 3D scaffold reinforcement. J. Biomed. Mater. Res. Part A. 94A:547–555, 2010.
Ammann, P. Strontium ranelate: a physiological approach for an improved bone quality. Bone. 38:15–18, 2006.
Assouline-Dayan, Y., C. Chang, A. Greenspan, Y. Shoenfeld, and M. E. Gershwin. Pathogenesis and natural history of osteonecrosis. Semin. Arthritis Rheum. 32:94–124, 2002.
Azarpira, N., M. Kaviani, and F. S. Sarvestani. Incorporation of VEGF-and bFGF-loaded alginate oxide particles in acellular collagen-alginate composite hydrogel to promote angiogenesis. Tissue Cell.72:101539, 2021.
Azimi-Nezhad, M. Vascular endothelial growth factor from embryonic status to cardiovascular pathology. Rep. Biochem. Mol. Biol. 2:59–69, 2014.
Balla, V. K., S. Bodhak, S. Bose, and A. Bandyopadhyay. Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater. 6:3349–3359, 2010.
Bandyopadhyay, A., A. Shivaram, S. Tarafder, H. Sahasrabudhe, D. Banerjee, and S. Bose. In vivo response of laser processed porous titanium implants for load-bearing implants. Ann Biomed Eng. 45:249–260, 2017.
Banerjee, S. S., S. Tarafder, N. M. Davies, A. Bandyopadhyay, and S. Bose. Understanding the influence of MgO and SrO binary doping on the mechanical and biological properties of beta-TCP ceramics. Acta Biomater. 6:4167–4174, 2010.
Bayer, E. A., J. Jordan, A. Roy, R. Gottardi, M. V. Fedorchak, P. N. Kumta, and S. R. Little. (*) Programmed platelet-derived growth factor-BB and bone morphogenetic protein-2 delivery from a hybrid calcium phosphate/alginate scaffold. Tissue Eng Part A. 23:1382–1393, 2017.
Bose, S., S. Tarafder, S. S. Banerjee, N. M. Davies, and A. Bandyopadhyay. Understanding in vivo response and mechanical property variation in MgO, SrO and SiO2 doped beta-TCP. Bone. 48:1282–1290, 2011.
Bose, S., S. Vahabzadeh, and A. Bandyopadhyay. Bone tissue engineering using 3D printing. Mater. Today. 16:496–504, 2013.
Brennan, M., P. Layrolle, and D. J. Mooney. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv. Funct. Mater. 2020. https://doi.org/10.1002/adfm.201909125.
Carlisle, E. M. In vivo requirement for silicon in articular cartilage and connective tissue formation in the chick. J. Nutr. 106:478–484, 1976.
Carmeliet, P., V. Ferreira, G. Breier, S. Pollefeyt, L. Kieckens, M. Gertsenstein, M. Fahrig, A. Vandenhoeck, K. Harpal, C. Eberhardt, C. Declercq, J. Pawling, L. Moons, D. Collen, W. Risau, and A. Nagy. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 380:435–439, 1996.
Cartland, S. P., S. W. Genner, A. Zahoor, and M. M. Kavurma. Comparative evaluation of TRAIL, FGF-2 and VEGF-A-induced angiogenesis in vitro and in vivo. Int. J. Mol. Sci. 17:2025, 2016.
Certelli, A., P. Valente, A. Uccelli, A. Grosso, N. Di Maggio, R. D’Amico, P. S. Briquez, J. A. Hubbell, T. Wolff, L. Gürke, E. Mujagic, R. Gianni-Barrera, and A. Banfi. Robust angiogenesis and arteriogenesis in the skin of diabetic mice by transient delivery of engineered VEGF and PDGF-BB proteins in fibrin hydrogels. Front Bioeng Biotechnol.9:688467, 2021.
Cha, H., S. Hong, J. H. Park, and H. H. Park. Stem cell-derived exosomes and nanovesicles: promotion of cell proliferation, migration, and anti-senescence for treatment of wound damage and skin ageing. Pharmaceutics. 2020. https://doi.org/10.3390/pharmaceutics12121135.
Chia, H. N., and B. M. Wu. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015. https://doi.org/10.1186/s13036-015-0001-4.
Cohen, D. L., E. Malone, H. Lipson, and L. J. Bonassar. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 12:1325–1335, 2006.
Dabrowski, B., W. Swieszkowski, D. Godlinski, and K. J. Kurzydlowski. Highly porous titanium scaffolds for orthopaedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 95:53–61, 2010.
Dai, J., and A. B. M. Rabie. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J. Dent. Res. 86:937–950, 2007.
Dashnyam, K., A. El-Fiqi, J. O. Buitrago, R. A. Perez, J. C. Knowles, and H. W. Kim. A mini review focused on the proangiogenic role of silicate ions released from silicon-containing biomaterials. J. Tissue Eng. 8:2041731417707339, 2017.
Dingsheng, L., L. Zengbing, and H. Dong. Favorable effects of progesterone on skin random flap survival in rats. Iran J. Basic Med. Sci. 19:1166–1170, 2016.
Dreyer, C. H., K. Kjaergaard, M. Ding, and L. Qin. Vascular endothelial growth factor for in vivo bone formation: a systematic review. J. Orthop. Translat. 24:46–57, 2020.
Druecke, D., S. Langer, E. Lamme, J. Pieper, M. Ugarkovic, H. U. Steinau, and H. H. Homann. Neovascularization of poly(ether ester) block-copolymer scaffolds in vivo: long-term investigations using intravital fluorescent microscopy. J. Biomed. Mater. Res. Part A. 68A:10–18, 2004.
Eftekhari, H., A. Jahandideh, A. Asghari, A. Akbarzadeh, and S. Hesaraki. Assessment of polycaprolacton (PCL) nanocomposite scaffold compared with hydroxyapatite (HA) on healing of segmental femur bone defect in rabbits. Artif. Cells Nanomed. Biotechnol. 45:961–968, 2017.
Ehrbar, M., V. G. Djonov, C. Schnell, S. A. Tschanz, G. Martiny-Baron, U. Schenk, J. Wood, P. H. Burri, J. A. Hubbell, and A. H. Zisch. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ. Res. 94:1124–1132, 2004.
Est-Witte, S. E., A. L. Farris, S. Y. Tzeng, D. L. Hutton, D. H. Gong, K. G. Calabresi, W. L. Grayson, and J. J. Green. Non-viral gene delivery of HIF-1α promotes angiogenesis in human adipose-derived stem cells. Acta Biomater. 113:279–288, 2020.
Fan, W., R. Crawford, and Y. Xiao. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials. 31:3580–3589, 2010.
Fei, Y., G. Gronowicz, and M. M. Hurley. Fibroblast growth factor-2, bone homeostasis and fracture repair. Curr. Pharm. Des. 19:3354–3363, 2013.
Ferrara, N., K. Carver-Moore, H. Chen, M. Dowd, L. Lu, K. S. O’Shea, L. Powell-Braxton, K. J. Hillan, and M. W. Moore. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 380:439–442, 1996.
Fielding, G. A., A. Bandyopadhyay, and S. Bose. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent. Mater. 28:113–122, 2012.
Franz, S., S. Rammelt, D. Scharnweber, and J. C. Simon. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 32:6692–6709, 2011.
Freeman, F. E., A. B. Allen, H. Y. Stevens, R. E. Guldberg, and L. M. McNamara. Effects of in vitro endochondral priming and pre-vascularisation of human MSC cellular aggregates in vivo. Stem Cell Res. Ther. 6:218, 2015.
Gao, G. F., A. F. Schilling, T. Yonezawa, J. Wang, G. H. Dai, and X. F. Cui. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol. J. 9:1304–1311, 2014.
Garcia, P., T. Histing, J. H. Holstein, M. Klein, M. W. Laschke, R. Matthys, A. Ignatius, B. Wildemann, J. Lienau, A. Peters, B. Willie, G. Duda, L. Claes, T. Pohlemann, and M. D. Menger. Rodent animal models of delayed bone healing and non-union formation: a comprehensive review. Eur. Cells Mater. 26:1–14, 2013.
Geng, Z., Z. Li, Z. Cui, J. Wang, X. Yang, and C. Liu. Novel bionic topography with MiR-21 coating for improving bone-implant integration through regulating cell adhesion and angiogenesis. Nano Lett. 20:7716–7721, 2020.
Grimes, D. R., C. Kelly, K. Bloch, and M. Partridge. A method for estimating the oxygen consumption rate in multicellular tumour spheroids. J. R. Soc. Interface. 2014. https://doi.org/10.1098/rsif.2013.1124.
Grosso, A., M. G. Burger, A. Lunger, D. J. Schaefer, A. Banfi, and N. Di Maggio. It takes two to tango: coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 5:68, 2017.
Gu, J. N., Q. Q. Zhang, M. R. Geng, W. Z. Wang, J. Yang, A. U. R. Khan, H. B. Du, Z. Sha, X. J. Zhou, and C. L. He. Construction of nanofibrous scaffolds with interconnected perfusable microchannel networks for engineering of vascularized bone tissue. Bioactive Mater. 6:3254–3268, 2021.
Guise, T. A. Bone loss and fracture risk associated with cancer therapy. Oncologist. 11:1121–1131, 2006.
Hankenson, K. D., M. Dishowitz, C. Gray, and M. Schenker. Angiogenesis in bone regeneration. Injury Int. J. Care Inj. 42:556–561, 2011.
He, X., Y. Liu, Y. Tan, L. M. Grover, J. Song, S. Duan, D. Zhao, and X. Tan. Rubidium-containing mesoporous bioactive glass scaffolds support angiogenesis, osteogenesis and antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 105:110155, 2019.
Ho-Shui-Ling, A., J. Bolander, L. E. Rustom, A. W. Johnson, F. P. Luyten, and C. Picart. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 180:143–162, 2018.
Hoppe, A., N. S. Gueldal, and A. R. Boccaccini. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 32:2757–2774, 2011.
Hoppe, A., N. S. Güldal, and A. R. Boccaccini. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 32:2757–2774, 2011.
Hu, K., and B. R. Olsen. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 126:509–526, 2016.
Huang, B., W. H. Wang, Q. C. Li, Z. Y. Wang, B. Yan, Z. M. Zhang, L. Wang, M. J. Huang, C. H. Jia, J. S. Lu, S. C. Liu, H. D. Chen, M. M. Li, D. Z. Cai, Y. Jiang, D. D. Jin, and X. C. Bai. Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone. Nat. Commun. 2016. https://doi.org/10.1038/ncomms13885.
Ibrahim, T., L. Mercatali, and D. Amadori. Bone and cancer: the osteoncology. Clin. Cases Miner. Bone Metab. 10:121–123, 2013.
Kang, M. S., J. H. Kim, R. K. Singh, J. H. Jang, and H. W. Kim. Therapeutic-designed electrospun bone scaffolds: mesoporous bioactive nanocarriers in hollow fiber composites to sequentially deliver dual growth factors. Acta Biomater. 16:103–116, 2015.
Kang, Y. Q., N. Mochizuki, A. Khademhosseini, J. Fukuda, and Y. Z. Yang. Engineering a vascularized collagen-beta-tricalcium phosphate graft using an electrochemical approach. Acta Biomater. 11:449–458, 2015.
Karageorgiou, V., and D. Kaplan. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 26:5474–5491, 2005.
Kargozar, S., F. Baino, S. Hamzehlou, R. G. Hill, and M. Mozafari. Bioactive glasses: sprouting angiogenesis in tissue engineering. Trends Biotechnol. 36:430–444, 2018.
Ke, Q., and M. Costa. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 70:1469–1480, 2006.
Kigami, R., S. Sato, N. Tsuchiya, N. Sato, D. Suzuki, Y. Arai, K. Ito, and B. Ogiso. Effect of basic fibroblast growth factor on angiogenesis and bone regeneration in non-critical-size bone defects in rat calvaria. J. Oral Sci. 56:17–22, 2014.
Kneser, U., E. Polykandriotis, J. Ohnolz, K. Heidner, L. Grabinger, S. Euler, K. U. Amann, A. Hess, K. Brune, P. Greil, M. Stürzl, and R. E. Horch. Engineering of vascularized transplantable bone tissues: induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 12:1721–1731, 2006.
Laschke, M. W., Y. Harder, M. Amon, I. Martin, J. Farhadi, A. Ring, N. Torio-Padron, R. Schramm, M. Rucker, D. Junker, J. M. Haufel, C. Carvalho, M. Heberer, G. Germann, B. Vollmar, and M. D. Menger. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 12:2093–2104, 2006.
Lee, J., D. Shin, and J. L. Roh. Use of a pre-vascularised oral mucosal cell sheet for promoting cutaneous burn wound healing. Theranostics. 8:5703–5712, 2018.
Lee, J. H., and H. W. Kim. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018. https://doi.org/10.1177/2041731418768285.
Lee, S. H., and H. Shin. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 59:339–359, 2007.
Lems, W. F., and H. G. Raterman. Critical issues and current challenges in osteoporosis and fracture prevention. An overview of unmet needs. Ther. Adv. Musculoskelet. Dis. 9:299–316, 2017.
Li, J. P., J. R. de Wijn, C. A. Van Blitterswijk, and K. de Groot. Porous Ti6Al4V scaffold directly fabricating by rapid prototyping: preparation and in vitro experiment. Biomaterials. 27:1223–1235, 2006.
Li, Y., Q. Pan, N. Zhang, B. Wang, Z. Yang, J. T. Ryaby, E. I. Waldorff, W.Y.-W. Lee, and G. Li. A novel pulsed electromagnetic field promotes distraction osteogenesis via enhancing osteogenesis and angiogenesis in a rat model. J. Orthop. Translat. 25:87–95, 2020.
Lichte, P., H. C. Pape, T. Pufe, P. Kobbe, and H. Fischer. Scaffolds for bone healing: concepts, materials and evidence. Injury Int. J. Care Inj. 42:569–573, 2011.
Lin, K., L. Xia, H. Li, X. Jiang, H. Pan, Y. Xu, W. W. Lu, Z. Zhang, and J. Chang. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials. 34:10028–10042, 2013.
Liu, Y., J. Fang, Q. Zhang, X. Zhang, Y. Cao, W. Chen, Z. Shao, S. Yang, D. Wu, M. Hung, Y. Zhang, W. Tong, and H. Tian. Wnt10b-overexpressing umbilical cord mesenchymal stem cells promote critical size rat calvarial defect healing by enhanced osteogenesis and VEGF-mediated angiogenesis. J. Orthop. Translat. 23:29–37, 2020.
Maes, C. Role and regulation of vascularization processes in endochondral bones. Calcif. Tissue Int. 92:307–323, 2013.
Maes, C., P. Carmeliet, K. Moermans, I. Stockmans, N. Smets, D. Collen, R. Bouillon, and G. Carmeliet. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech. Dev. 111:61–73, 2002.
Maes, C., I. Stockmans, K. Moermans, R. Van Looveren, N. Smets, P. Carmeliet, R. Bouillon, and G. Carmeliet. Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. J. Clin. Investig. 113:188–199, 2004.
Maggiore, U., R. Oberbauer, J. Pascual, O. Viklicky, C. Dudley, K. Budde, S. S. Sorensen, M. Hazzan, M. Klinger, and D. Abramowicz. Strategies to increase the donor pool and access to kidney transplantation: an international perspective. Nephrol. Dial. Transplant. 30:217–222, 2015.
Maier, J. A., D. Bernardini, Y. Rayssiguier, and A. Mazur. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta. 1689:6–12, 2004.
Marchand, M., C. Monnot, L. Muller, and S. Germain. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin. Cell Dev. Biol. 89:147–156, 2019.
Mehdizadeh, H., E. S. Bayrak, C. Lu, S. I. Somo, B. Akar, E. M. Brey, and A. Cinar. Agent-based modeling of porous scaffold degradation and vascularization: optimal scaffold design based on architecture and degradation dynamics. Acta Biomater. 27:167–178, 2015.
Mercado-Pagán, Á. E., A. M. Stahl, Y. Shanjani, and Y. Yang. Vascularization in bone tissue engineering constructs. Ann. Biomed. Eng. 43:718–729, 2015.
Mitra, I., S. Bose, W. S. Dernell, N. Dasgupta, C. Eckstrand, J. Herrick, M. J. Yaszemski, S. B. Goodman, and A. Bandyopadhyay. 3D Printing in alloy design to improve biocompatibility in metallic implants. Mater. Today (Kidlington). 45:20–34, 2021.
Pajarinen, J., T. Lin, E. Gibon, Y. Kohno, M. Maruyama, K. Nathan, L. Lu, Z. Yao, and S. B. Goodman. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 196:80–89, 2019.
Pandya, M., M. Saxon, J. Bozanich, C. Tillberg, X. Luan, and T. G. H. Diekwisch. The glycoprotein/cytokine erythropoietin promotes rapid alveolar ridge regeneration in vivo by promoting new bone extracellular matrix deposition in conjunction with coupled angiogenesis/osteogenesis. Int. J. Mol. Sci. 2021. https://doi.org/10.3390/ijms22062788.
Patel, Z. S., S. Young, Y. Tabata, J. A. Jansen, M. E. Wong, and A. G. Mikos. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 43:931–940, 2008.
Paul, J. D., K. L. K. Coulombe, P. T. Toth, Y. Zhang, G. Marsboom, V. P. Bindokas, D. W. Smith, C. E. Murry, and J. Rehman. SLIT3-ROBO4 activation promotes vascular network formation in human engineered tissue and angiogenesis in vivo. J. Mol. Cell Cardiol. 64:124–131, 2013.
Pérez, R. A., J. E. Won, J. C. Knowles, and H. W. Kim. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 65:471–496, 2013.
Post, M. J., R. Laham, F. W. Sellke, and M. Simons. Therapeutic angiogenesis in cardiology using protein formulations. Cardiovasc. Res. 49:522–531, 2001.
Qian, B., Q. Yang, M. Wang, S. Huang, C. Jiang, H. Shi, Q. Long, M. Zhou, Q. Zhao, and X. Ye. Encapsulation of lyophilized platelet-rich fibrin in alginate-hyaluronic acid hydrogel as a novel vascularized substitution for myocardial infarction. Bioact. Mater. 7:401–411, 2022.
Quade, M., S. Knaack, D. Weber, U. König, B. Paul, P. Simon, A. Rösen-Wolff, R. Schwartz-Albiez, M. Gelinsky, and A. Lode. Heparin modification of a biomimetic bone matrix modulates osteogenic and angiogenic cell response in vitro. Eur. Cell Mater. 33:105–120, 2017.
Rademakers, T., J. M. Horvath, C. A. van Blitterswijk, and V. L. S. LaPointe. Oxygen and nutrient delivery in tissue engineering: approaches to graft vascularization. J. Tissue Eng. Regen. Med. 13:1815–1829, 2019.
Ramasamy, S. K., A. P. Kusumbe, L. Wang, and R. H. Adams. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 507:376–380, 2014.
Rezwan, K., Q. Z. Chen, J. J. Blaker, and A. R. Boccaccini. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 27:3413–3431, 2006.
Rivron, N. C., J. J. Liu, J. Rouwkema, J. de Boer, and C. A. van Blitterswijk. Engineering vascularised tissues in vitro. Eur. Cell Mater. 15:27–40, 2008.
Rong, Q., S. Li, Y. Zhou, Y. Geng, S. Liu, W. Wu, T. Forouzanfar, G. Wu, Z. Zhang, and M. Zhou. A novel method to improve the osteogenesis capacity of hUCMSCs with dual-directional pre-induction under screened co-culture conditions. Cell Prolif.53:e12740, 2020.
Rouwkema, J., N. C. Rivron, and C. A. van Blitterswijk. Vascularization in tissue engineering. Trends Biotechnol. 26:434–441, 2008.
Rutanen, J., T. T. Rissanen, A. Kivelä, I. Vajanto, and S. Ylä-Herttuala. Clinical applications of vascular gene therapy. Curr. Cardiol. Rep. 3:29–36, 2001.
Santos, L., G. Fuhrmann, M. Juenet, N. Amdursky, C. M. Horejs, P. Campagnolo, and M. M. Stevens. Extracellular stiffness modulates the expression of functional proteins and growth factors in endothelial cells. Adv. Healthc. Mater. 4:2056–2063, 2015.
Saridis, A., E. Panagiotopoulos, M. Tyllianakis, C. Matzaroglou, N. Vandoros, and E. Lambiris. The use of the Ilizarov method as a salvage procedure in infected nonunion of the distal femur with bone loss. J. Bone Joint Surg. Br. 88B:232–237, 2006.
Savkovic, V., H. Li, D. Obradovic, F. F. Masieri, A. K. Bartella, R. Zimmerer, J. C. Simon, C. Etz, and B. Lethaus. The angiogenic potential of mesenchymal stem cells from the hair follicle outer root sheath. J. Clin. Med. 2021. https://doi.org/10.3390/jcm10050911.
Sen, C. K., S. Khanna, M. Venojarvi, P. Trikha, E. C. Ellison, T. K. Hunt, and S. Roy. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Heart Circ. Physiol. 282:H1821-1827, 2002.
Shi, Y., G. Kramer, A. Schröder, C. J. Kirkpatrick, A. Seekamp, H. Schmidt, and S. Fuchs. Early endothelial progenitor cells as a source of myeloid cells to improve the pre-vascularisation of bone constructs. Eur. Cell Mater. 27:64–79, 2014. (discussion 79–80)
Silva, A. S., L. F. Santos, M. C. Mendes, and J. F. Mano. Multi-layer pre-vascularized magnetic cell sheets for bone regeneration. Biomaterials.231:119664, 2020.
Somo, S. I., B. Akar, E. S. Bayrak, J. C. Larson, A. A. Appel, H. Mehdizadeh, A. Cinar, and E. M. Brey. Pore interconnectivity influences growth factor-mediated vascularization in sphere-templated hydrogels. Tissue Eng. Part C Methods. 21:773–785, 2015.
Sozen, T., L. Ozisik, and N. C. Basaran. An overview and management of osteoporosis. Eur. J. Rheumatol. 4:46–56, 2017.
Subbiah, R., and R. E. Guldberg. Materials science and design principles of growth factor delivery systems in tissue engineering and regenerative medicine. Adv. Healthc. Mater. 8:e1801000, 2019.
Subbiah, R., C. Hipfinger, A. Tahayeri, A. Athirasala, S. Horsophonphong, G. Thrivikraman, C. M. França, D. A. Cunha, A. Mansoorifar, A. Zahariev, J. M. Jones, P. G. Coelho, L. Witek, H. Xie, R. E. Guldberg, and L. E. Bertassoni. 3D printing of microgel-loaded modular microcages as instructive scaffolds for tissue engineering. Adv. Mater. 32:e2001736, 2020.
Subbiah, R., M. P. Hwang, S. Y. Van, S. H. Do, H. Park, K. Lee, S. H. Kim, K. Yun, and K. Park. Osteogenic/angiogenic dual growth factor delivery microcapsules for regeneration of vascularized bone tissue. Adv. Healthc. Mater. 4:1982–1992, 2015.
Sun, Y., X. Jiang, Y. Liu, D. Liu, C. Chen, C. Lu, S. Zhuang, A. Kumar, and J. Liu. Recent advances in Cu(II)/Cu(I)-MOFs based nano-platforms for developing new nano-medicines. J. Inorg. Biochem.225:111599, 2021.
Sun, X., Y. Kang, J. Bao, Y. Zhang, Y. Yang, and X. Zhou. Modeling vascularized bone regeneration within a porous biodegradable CaP scaffold loaded with growth factors. Biomaterials. 34:4971–4981, 2013.
Tarafder, S., V. K. Balla, N. M. Davies, A. Bandyopadhyay, and S. Bose. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J. Tissue Eng. Regen. Med. 7:631–641, 2013.
Thomlinson, R. H., and L. H. Gray. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer. 9:539–549, 1955.
Thummarati, P., and M. Kino-Oka. Exogenous FGF-2 prolongs endothelial connection in multilayered human skeletal muscle cell sheet. J. Biosci. Bioeng. 131:686–695, 2021.
Vasvári, G. F., D. Csonka, T. Zsebe, Á. Schiffer, I. Samardžić, R. Told, A. Péntek, and P. Maróti. CMT additive manufacturing parameters defining aluminium alloy object geometry and mechanical properties. Materials (Basel). 2021. https://doi.org/10.3390/ma14061545.
Walker, J., S. Shadanbaz, T. B. Woodfield, M. P. Staiger, and G. J. Dias. Magnesium biomaterials for orthopedic application: a review from a biological perspective. J. Biomed. Mater. Res. B Appl. Biomater. 102:1316–1331, 2014.
Walthers, C. M., A. K. Nazemi, S. L. Patel, B. M. Wu, and J. C. Dunn. The effect of scaffold macroporosity on angiogenesis and cell survival in tissue-engineered smooth muscle. Biomaterials. 35:5129–5137, 2014.
Wang, M., Y. Yu, K. Dai, Z. Ma, Y. Liu, J. Wang, and C. Liu. Improved osteogenesis and angiogenesis of magnesium-doped calcium phosphate cement via macrophage immunomodulation. Biomater. Sci. 4:1574–1583, 2016.
Wascher, D. C., and L. Bulthuis. Extremity trauma: field management of sports injuries. Curr. Rev. Musculoskelet. Med. 7:387–393, 2014.
Wilson, C. E., J. D. de Bruijn, C. A. van Blitterswijk, A. J. Verbout, and W. J. A. Dhert. Design and fabrication of standardized hydroxyapatite scaffolds with a defined macro-architecture by rapid prototyping for bone-tissue-engineering research. J. Biomed. Mater. Res. Part A. 68A:123–132, 2004.
Wood, K. J., and R. Goto. Mechanisms of rejection: current perspectives. Transplantation. 93:1–10, 2012.
Woodruff, M. A., and D. W. Hutmacher. The return of a forgotten polymer—polycaprolactone in the 21st century. Progress Polym. Sci. 35:1217–1256, 2010.
Wu, C., Y. Zhou, W. Fan, P. Han, J. Chang, J. Yuen, M. Zhang, and Y. Xiao. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials. 33:2076–2085, 2012.
Wu, C., Y. Zhou, M. Xu, P. Han, L. Chen, J. Chang, and Y. Xiao. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 34:422–433, 2013.
Xie, H., Z. Cui, L. Wang, Z. Xia, Y. Hu, L. Xian, C. Li, L. Xie, J. Crane, M. Wan, G. Zhen, Q. Bian, B. Yu, W. Chang, T. Qiu, M. Pickarski, L. T. Duong, J. J. Windle, X. Luo, E. Liao, and X. Cao. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 20:1270–1278, 2014.
Xie, K., L. Wang, Y. Guo, S. Zhao, Y. Yang, D. Dong, W. Ding, K. Dai, W. Gong, G. Yuan, and Y. Hao. Effectiveness and safety of biodegradable Mg-Nd-Zn-Zr alloy screws for the treatment of medial malleolar fractures. J. Orthop. Translat. 27:96–100, 2021.
Xu, R., A. Yallowitz, A. Qin, Z. Wu, D. Y. Shin, J. M. Kim, S. Debnath, G. Ji, M. P. Bostrom, X. Yang, C. Zhang, H. Dong, P. Kermani, S. Lalani, N. Li, Y. Liu, M. G. Poulos, A. Wach, Y. Zhang, K. Inoue, A. Di Lorenzo, B. Zhao, J. M. Butler, J. H. Shim, L. H. Glimcher, and M. B. Greenblatt. Targeting skeletal endothelium to ameliorate bone loss. Nat. Med. 24:823–833, 2018.
Yang, F., D. Yang, J. Tu, Q. Zheng, L. Cai, and L. Wang. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells. 29:981–991, 2011.
Yu, W. L., T. W. Sun, C. Qi, H. K. Zhao, Z. Y. Ding, Z. W. Zhang, B. B. Sun, J. Shen, F. Chen, Y. J. Zhu, D. Y. Chen, and Y. H. He. Enhanced osteogenesis and angiogenesis by mesoporous hydroxyapatite microspheres-derived simvastatin sustained release system for superior bone regeneration. Sci. Rep. 7:44129, 2017.
Yun, Y. R., J. E. Won, E. Jeon, S. Lee, W. Kang, H. Jo, J. H. Jang, U. S. Shin, and H. W. Kim. Fibroblast growth factors: biology, function, and application for tissue regeneration. J. Tissue Eng. 2010:218142, 2010.
Zhang, Z., B. Jia, H. Yang, Y. Han, Q. Wu, K. Dai, and Y. Zheng. Biodegradable ZnLiCa ternary alloys for critical-sized bone defect regeneration at load-bearing sites: In vitro and in vivo studies. Bioact. Mater. 6:3999–4013, 2021.
Zhang, Y., Y. Ma, C. Wu, R. J. Miron, and X. Cheng. Platelet-derived growth factor BB gene-released scaffolds: biosynthesis and characterization. J. Tissue Eng. Regen. Med. 10:E372-e381, 2016.
Zhang, J., J. Pan, and W. Jing. Motivating role of type H vessels in bone regeneration. Cell Prolif. 53:e12874, 2020.
Zhang, L., G. Yang, B. N. Johnson, and X. Jia. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 84:16–33, 2019.
Zhao, F., B. Lei, X. Li, Y. Mo, R. Wang, D. Chen, and X. Chen. Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes. Biomaterials. 178:36–47, 2018.
Zhao, H., S. Shen, L. Zhao, Y. Xu, Y. Li, and N. Zhuo. 3D printing of dual-cell delivery titanium alloy scaffolds for improving osseointegration through enhancing angiogenesis and osteogenesis. BMC Musculoskelet. Disord. 22:734, 2021.
Zhou, L., Y. Han, J. Ding, X. Chen, S. Huang, X. Xing, D. Wu, and J. Chen. Regulation of an antimicrobial peptide GL13K-modified titanium surface on osteogenesis, osteoclastogenesis, and angiogenesis base on osteoimmunology. ACS Biomater. Sci. Eng. 7:4569–4580, 2021.
Funding
The author(s) received partial financial support for the research by Jiangsu Commission of Health M2020025.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Additional information
Associate Editor Michael S. Detamore oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, J., Han, Q., Cai, M. et al. Effect of Angiogenesis in Bone Tissue Engineering. Ann Biomed Eng 50, 898–913 (2022). https://doi.org/10.1007/s10439-022-02970-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-022-02970-9