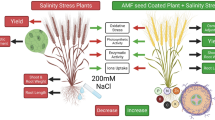
Abstract
Nowadays, there has been an increasing demand for organic agricultural products as biofertilizer that are safe for humans, livestock, environment and economically viable to reduce the negative effect of stresses on plants and stimulate crop production. Salinity is a major issue that adversely affects plant growth and productivity. The current study was carried out to investigate changes in the growth, biochemical parameters, and yield of wheat plant in response to arbuscular mycorrhizal fungi (AMF) at 1% and two levels of yeast extract at 1% and 2% under the effect of salinity levels at 4 dS/m and 8 dS/m. Results show that salinity stress caused significant decreases in vegetative growth parameters, photosynthetic pigments, indole acetic acid (IAA), grains yield/plant, carbohydrate content accompanied by significant increases in two osmolytes (total soluble sugars and proline), hydrogen peroxide (H2O2), malondialdehyde (MDA), antioxidant enzymes (Peroxidase, POD), superoxide dismutase (SOD), catalase (CAT), some antioxidant substances of the yielded grains total phenolic, flavonoid, lycopene, β‑carotene and antioxidant activity (DPPH). The deleterious effect of salinity at 8 dS/m was more than that of salinity at 4 dS/m. Since, salinity at 4 dS/m caused non significant decreases in grains yield/plant by 8.25%. Whereas, salinity at 8 dS/m significantly decreased grains yield/plant by 15.73% relative to control. Nevertheless, all applied treatments (AMF, yeast extract at 1% and 2%) alleviated the deleterious effect of both salinity levels (4 dS/m and 8 dS/m) by improving vegetative growth parameters, photosynthetic pigments, IAA, total soluble sugars and proline, H2O2, MDA, antioxidant enzymes (POD, SOD, CAT), grains yield/plant, and some biochemical constituents of the yielded grains. The most pronounced treatment was 2% yeast extract that increased shoot dry weight/plant by 48.33% under 4 dS/m salinity level and by 69.32% under 8 dS/m salinity level relative to corresponding controls. In addition, AMF, 1% yeast extract, and 2% yeast extract significantly increased grains yield/plant by 17.89%, 24.12%, and 35.41% respectively under 8 dS/m salinity stress relative to control. It could be concluded that the promotive effect of yeast extract at 2% > yeast extract at 1% > AMF in increasing wheat salinity tolerance and decreasing harmful effect of salinity on wheat growth, grain yield quality and quantity.


Similar content being viewed by others
References
Abbas SM (2013) The influence of biostimulants on the growth and on the biochemical composition of Vicia faba cv. Giza 3 beans. Rom Biotech Lett 18(2):8061–8080
Abdelaal KA, Hafez YM, El Sabagh A, Saneoka H (2017) Ameliorative effects of abscisic acid and yeast on morpho-physiological and yield characteristics of maize plant (Zea mays L.) under water deficit conditions. Fresenius Environ Bull 26:7372–7383
Abou EL-Yazied A, Mady MA (2012) Effect of boron and yeast extract foliar application on growth, pod setting and both green pod and seed yield of broad bean (Vicia faba L). J Appl Sci Res 8(2):1240–1251
Ait-El-Mokhtar M, Laouane RB, Anli M, Boutasknit A, Wahbi S, Meddich A (2019) Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Sci Hortic 253:429–438. https://doi.org/10.1016/j.scienta.2019.04.066
Al-Ashkar I, Alderfasi A, Ben Romdhane W, Seleiman MF, El-Said RA, Al-Doss A (2020) Morphological and genetic diversity within salt tolerance detection in eighteen wheat genotypes. Plants 9:287
Albalasmeh AA, Berhe AA, Ghezzehei TA (2013) A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr Polym 97(2):253–261. https://doi.org/10.1016/j.carbpol.2013.04.072
Asghari HR, Marschner P, Smith SE, Smith FA (2005) Growth response of Atriplexnummulariato inoculation with arbuscularmycorrhizal fungi at different salinity levels. Plt Soil 273:245–256
Ashraf M, Foolad MR (2007) Roles of glycinebetaine and proline in improving plant abiotic stress tolerance. Environ Exp Bot 59:206–216
Ashraf M, Harris P (2003) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166(024):3–16. https://doi.org/10.1016/j.plant
Atkinson NJ, Urwin PE (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot 63:3523–3543
Awad-Allah EFA, Attia MG, Mahdy AM (2020) Salinity stress alleviation by foliar bio-stimulant, proline and potassium nutrition promotes growth and yield quality of garlic plant. Open J Soil Sci 10:443–458. https://doi.org/10.4236/ojss.2020.109023
Barea J, Estrada B, Aroca R, Maathuis J, Ruiz-Lozano J (2013) Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerant to salinity through improved ion homoestasis. Plant Cell Environ 36:1771–1782
Barnett JA, Payne RW, Yarrow D (eds) (1990) Yeasts: characterization and identification. Cambridge University Press, Cambridge
Bates LS, Waldan RP, Teare LD (1973) Rapid determination of free proline under water stress studies. Plant Soil 39:205–207
Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, Ahmed N, Zhang L (2019) Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front Plant Sci 10:1068. https://doi.org/10.3389/fpls.2019.01068
Chanclud E, Moral JB (2016) Plant hormones: A fungal point of view. Mol Plant Pathol 17(8):1289–1297. https://doi.org/10.1111/mpp.12393
Chandran AKN, Kim JW, Yoo YH, Park HL, Kim YJ, Cho MH, Jung KH (2019) Transcriptome analysis of rice-seedling roots under soil-salt stress using RNA-Seq method. Plant Biotechnol Rep 13:567–578
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Chaparzadeh N, Amico MLD, Khavari-Najad RA, Izzo R, Navarizzo F (2004) Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiol Biochem 42:695–701
Chen JX, Wang XF (2006) Plant physiology experimental guide. Higher Education, Beijing, pp 24–56
Chinnusamy V, Jagendorf A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448. https://doi.org/10.2135/cropsci2005.0437
Colla G, Rouphael Y, Cardarelli M, Tullio M, Rivera C, Rea E (2008) Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentration. Biol Fertil Soils 44:501–509
Conversa G, Lazzizera C, Chiaravalle AE, Miedico O, Bonasia A, La Rotonda P, Elia A (2019) Selenium fern application and arbuscular mycorrhizal fungi soil inoculation enhance Se content and antioxidant properties of green asparagus (Asparagus offcinalis L.) spears. Sci Hortic 252:176–191
Darwesh RSS (2016) Phoenix dactylifera cv. Medjol plantlets as affected by yeast extract and NPK fertilizers. Ann Agric Environ Sci 1:7–14
Dawood MG, El-Lethy SR, Sadak Mh (2013) Role of Methanol and yeast in improving growth, yield, nutritive value and antioxidants of soybean. World Appl Sci J 26(1):6–14
De Pascale S, Maggio A, Fogliano V, Abrosino P, Ritieni A (2001) Irrigation with saline water improves carotenoids content and antioxidant activity of tomato. J Hortic Sci Biotechnol 76(4):447–453
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulator in plants. Plant J 4:215–223
Dumas Y, Dadomo M, Di lucca G, Grolier P (2003) Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J Sci Food Agric 83:369–382
El-Bially MA, Saudy HS, El-Metwally IM, Shahin MG (2022) Sunflower response to application of L‑ascorbate under thermal stress associated with different sowing dates. Gesunde Pflanz 74:87–96. https://doi.org/10.1007/s10343-021-00590-2
El-Metwally IM, Saudy HS, Abdelhamid MT (2021) Efficacy of benzyladenine for compensating the reduction in soybean productivity under low water supply. Ital J Agrometeorol 2:81–90. https://doi.org/10.36253/ijam-872
El-Motty AEZ, Shahin MFM, El-Shiekh MH, Abd-El-Migeed MMM (2010) Effect of algae extract and yeast application on growth, nutritional status, yield and fruit quality of Keitte mango trees. ABJNA 1:421–429
Emam MM (2013) Efficiency of yeast in enhancement of the oxidative defense system in salt-stressed flax seedlings. Acta Biol Hung 64(1):118–130. https://doi.org/10.1556/ABiol.64.2013.1.11
Ezz El-Din AA, Hendawy SF (2010) Effect of dry yeast and tea compost on growth and oil content of Borago officinalis plant. Res J Agric Biol Sci 6(4):424–430
Giri B, Kapoor R, Mukerji KG (2007) Improved tolerance of Acacia nilotica to salt stress by Arbuscular Mycorrhiza, Glomusfasciculatummay be partly related to elevated K/NA ratios in root and shoot tissues. Microb Ecol 54:753–760
Gonzalez M, Guzman B, Rudkyk R, Romano E, Molina MAA (2003) Spectrophotometric determination of phenolic compounds in PropolisLat. Am J Pharm 22(3):243–248
Guo R, Yang Z, Li F, Yan C, Zhong X et al (2015) Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum L.) to salt and alkali stress. BMC Plant Biol 15:170. https://doi.org/10.1186/s12870-015-0546-x
Gusmiaty A, Restu M, Payangan RY (2019) Production of IAA (indole acetic acid) of rhizosphere fungus in the suren community forest stand. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/343/1/012058
Gyamfi MA, Yonamine M, Aniya Y (2002) Free radical scavenging action of medicinal herbs from GhanaThonningia sanguine on experimentally induced liver injuries. Gen Pharmacol 32:661–667
Haider I, Raza MAS, Iqbal R, Ahmad S, Aslam MU, Israr M, Riaz U, Sarfraz M, Abbas N, Abbasi SH, Abbas Z, Aamer M (2021) Alleviating the drought stress in wheat (Triticum aestivum L.) by foliar application of amino acid and yeast. Pak J Agric Res 34(1):239–246. https://doi.org/10.17582/journal.pjar/2021/34.1.239.246
Hamed SM, El-Gaml NM, Eissa ST (2022) Integrated biofertilization using yeast with cyanobacteria on growth and productivity of wheat. Beni-Suef Univ J Basic Appl Sci 11:112. https://doi.org/10.1186/s43088-022-00288-y
Han RM, Zhang JP, Skibsted LH (2012) Reaction dynamics of flavonoids andcarotenoidsas antioxidants. Molecules 17:2140–2160. https://doi.org/10.3390/molecules17022140
Hasanuzzaman M, Nahar K, Fujita M, Ahmad P, Chandna R et al (2013) Enhancing plant productivity under salt stress: Relevance of poly-omics. In: Ahmad P, Azooz MM, Prasad MNV (eds) Salt stress in plants: Omics, signaling and responses. Springer, Berlin, pp 113–156 https://doi.org/10.1007/978-1-4614-6108-1_6
Hasanuzzaman M, Nahar K, Rahman A, Anee TI, Alam MU et al (2017) Approaches to enhance salt stress tolerance in wheat. Intech Open, London, pp 151–187 https://doi.org/10.5772/67247
Herbert DP, Phipps J, Strange RE (1971) Chemical analysis of microbial cells. Methods Microbiol 5:209–344. https://doi.org/10.1016/S0580-9517(08)70641-X
Hodges DM, De Long JM, Forney CF, Prange RK (1999) Improving the thiobarbaturic acid reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Homme PM, Gonzalez B, Billard J (1992) Carbohydrate content, frutane and sucrose enzyme activities in roots, stubble and leaves of rye grass (Lolium perenne L.) as affected by sources/link modification after cutting. J Plant Physiol 140:282–291
Igiehon ON, Babalola OO (2021) Rhizobium and Mycorrhizal fungal species improved soybean yield under drought stress conditions. Curr Microbiol 78:1615–1627
Jajoo A, Mathur S (2021) Role of arbuscular mycorrhizal fungi as an underground saviuor for protecting plants from abiotic stresses. Physiol Mol Biol Plants 27(11):2589–2603. https://doi.org/10.1007/s12298-021-01091-2
Jansa J, Forczek ST, Rozmoš M, Püschel D, Bukovská P, Hršelová H (2019) Arbuscular mycorrhiza and soil organic nitrogen: Network of players and interactions. Chem Biol Technol Agric. https://doi.org/10.1186/s40538-019-0147-2
Kaur G, Asthir B (2015) Proline: a key player in plant abiotic stress tolerance. Biol Plant 59:609–619
Kazan K (2013) Auxin and the integration of environmental signals into plant root development. Ann Bot 112(9):1655–1665. https://doi.org/10.1093/aob/mct229
Kerepesi I, Galiba G (2000) Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci 40(2):482–487
Khalil SE, Ismael EG (2010) Growth, yield and seed quality of lupinustermis as affected by different soil moisture levels and different ways of yeast application. J Am Sci 6(8):141–153
Kivlin SN, Hawkes CV, Treseder KK (2011) Global diversity and distribution of arbuscular mycorrhizal fungi. Soil Biol Biochem 43:2294–2303
Kong FX, Hu W, Chao WL, Sang WL, Wang LS (1999) Physiological responses of Mexicana to oxidative stress of SO2. Environ Exp Bot 42:201–209
Krauss S, Schnitzler WH, Grassmann J, Woitke M (2006) The influence of different electrical conductivity values in a simplified recirculating soilless system on inner and outer fruit quality characteristics of tomato. J Agric Food Chem 54:441–448. https://doi.org/10.1021/jf051930a
Kumar KB, Khan PA (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusinecoracana cv. PR 202) leaves during senescence. Indian J Exp Bot 20:412–416
Li Z, Wu N, Meng S, Wu F, Liu T (2020) Arbuscular mycorrhizal fungi (AMF) enhance the tolerance of EuonymusmaackiiRupr at a moderate level of salinity. PLoS ONE 15:e231497. https://doi.org/10.1371/journal.pone.0231497
Liao X, Chen J, Guan R, Liu J, Sun Q (2021) Two arbuscular mycorrhizal fungi alleviates drought stress and improves plant growth in cinnamomum migao seedlings. Mycobiology 49(4):396–405
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P (eds) Current protocols in food analytical chemistry (CPFA). Wiley, New York, pp F4.3.1–F4.3.8
Mady MA (2009) Effect of foliar application with yeast extract and zinc on fruit setting and yield of faba bean (Vicia fabaL.). J Biol Chem Environ Sci 4(2):109–127
Makhlouf BSI, Khalil SRA, Saudy HS (2022) Efficacy of humic acids and chitosan for enhancing yield and sugar quality of sugar beet under moderate and severe drought. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-022-00762-7
Mansour E, Moustafa ESA, Desoky ESM, Ali MMA, Yasin MAT, Attia A, Alsuhaibani N, Tahir MU, El-Hendawy S (2020) Multidimensional evaluation for detecting salt tolerance of bread wheat genotypes under actual saline field growing conditions. Plants 9:1324
Marzauk NM, Shafeek MR, Helmy YI, Ahmed AA, Shalaby M (2014) Effect of vitamin E and yeast extract foliar application on growth, pod yield and both green pod and seed yield of broad bean (Vicia faba L.). Mid East J Appl Sci 4(1):61–67
Mostafa EAM, Abou Raya MS (2003) Effect of soil application of active dry yeast on growth, yield and fruit quality of Grand Nain banana cv. Arab Univ J Agric Sci 12(2):693–704
Muhsin M, Nawaz M, Khan I, Chattha MB, Khan S et al (2021) Efficacy of seed size to improve field performance of wheat under late sowing conditions. Pak J Agric Res 34:247–254
Musyoka DM, Njeru EM, Nyamwange MM, Maingi JM (2020) Arbuscular mycorrhizal fungi and Bradyrhizobium co-inoculation enhances nitrogen fixation and growth of green grams (Vigna radiate L.) under water stress. J Plant Nutr 43(7):1036–1047. https://doi.org/10.1080/01904167.2020.1711940.43
Ordoñez AAL, Gomez JD, Vattuone MA, Lsla MI (2006) Antioxidant activities of Sechiumedule(Jacq.) Swartz extracts. Food Chem 97:452–458. https://doi.org/10.1016/j.foodchem.2005.05.024
Parihar P, Bora M (2018) Effect of mycorrhiza (Glomusmosseae) on morphological and biochemical properties of Ashwagandha (Withaniasomnifera) (L.)Dunal. J Appl Nat Sci 10:1115–1123
Payyavula RS, Navarre DA, Kuhl JC, Pantoja A, Pillai SS (2012) Differential effects of environment on potato phenylpropanoid and carotenoid expression. BMC Plant Biol 12(39):1–17. https://doi.org/10.1186/1471-2229-12-39
Pedranzani H, Rodríguez-Rivera M, Gutierrez M, Porcel R, Hause B, Ruiz-Lozano JM (2015) Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitariaerianthaplants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 26:141–152
Quamruzzaman M, Manik SMN, Shabala S, Zhou M (2021) Improving performance of salt-grown crops by exogenous application of plant growth regulators. Biomolecules 11:788. https://doi.org/10.3390/biom11060788
Rady MM, Taha RS, Semida WM, Alharby HF (2017) Modulation of salt stress effects on Vicia faba L. plants grown on a reclaimed-saline soil by salicylic acid application. Rom Agric Res 34:175–185
Ragaey MM, Sadak MS, Dawood MFA, Mousa NHS, Hanafy RS, Latef AAHA (2022) Role of signaling molecules sodium nitroprusside and arginine in alleviating salt-induced oxidative sStress in wheat. Plants 11:1786. https://doi.org/10.3390/plants11141786
Robin AHK, Matthew C, Uddin MJ, Bayazid KN (2016) Salinity induced reduction in root surface area and changes in major root and shoot traits at the phytomer level in wheat. J Exp Bot 67:3719–3729
Sadak MS (2016) Physiological role of yeast extract and nicotinamide on Pisum sativumL. plants under heat stress. Int J Pharm Tech Res 9(9):170–178
Sadak MS (2022) Biochemical responses of white termis to pyridoxine and mycorrhizae treatment under salinity stress. Egypt J Chem 65(10):429–439. https://doi.org/10.21608/EJCHEM.2022.118032.5319
Sadak MS, Ahmed MMR (2016) Physiological role of cyanobacteria and glycinebetaine on wheat plant grown under salinity stress. Int J Pharm Tech Res 9(7):78–92
Sharma MP, Grover M, Chourasiya D, Bharti A, Agnihotri R, Maheshwari HS, Pareek A, Buyer JS, Sharma SK, Schütz L (2020) Deciphering the role of trehalose in tripartite symbiosis among rhizobia, arbuscular mycorrhizal fungi, and legumes for enhancing abiotic stress tolerance in crop plants. Front Microbiol 11:509919. https://doi.org/10.3389/fmicb.2020.509919
Sheng M, Tang M, Zhang F, Huang Y (2011) Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 21:423–430. https://doi.org/10.1007/s00572-010-0353-z
Smith FA, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62:227–250
Smith SE, Read DJ (2010) Mycorrhizal symbiosis. Academic Press, Cambridge
Snedecor GW, Cochran WG (1980) Statistical methods, 7th edn. Iowa State Univ. Press, Iowa
Sofy M, Mohamed H, Dawood M, Abu-Elsaoud A, Soliman M (2021) Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Arch Agron Soil Sci 21:1–22
Stewart LI, Hamel C, Hogue R, Moutoglis P (2005) Response of strawberry to inoculation with arbuscular myccorrhizal fungi under very high soil phosphorus conditions. Mycorrhiza 15:612–619
Taha S, Seleiman R, Alhammad MF, Alkahtani BA, Alwahibi J, Mahdi MS (2021) activated yeast extract enhances growth, anatomical structure, and productivity of Lupinus termis L. plants under actual salinity conditions. Agronomy 11:74. https://doi.org/10.3390/agronomy11010074
Talaat NB, Shawky B (2014) Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot 98:20–31
USDA (United States Department of Agriculture), Foreign agricultural services (2021) Egypt is able to secure a steady supply of grains during the COVID-19 pandemic, grain and feed annual, gain global agricultural information network, report number EG2021–0004
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci 5:59–66
Wang Y, Li X, Li J, Bao Q, Zhang F, Tulaxi G, Wang Z (2016) Salt-induced hydrogen peroxide is involved in modulation of antioxidant enzymes in cotton. Crop J 4:490–498
Wen-Ya MA, Qiang-Sheng WU, Yong-Jie XU, Kamil K (2021) Exploring mycorrhizal fungi in walnut with a focus on physiological roles. Not Bot Horti Agrobot 49:12363
Wu QS, Ying-Ning Z, Abd-Allah EF (2014) Mycorrhizal association and ROS in plants. In: Ahmad P (ed) Oxidative damage to plants. Academic Press, New York, pp 453–475
Xue FF, Liu L, Liu ZP, Mehta SK, Zhao GM (2008) Protective role of Ca against NaCl toxicity in Jerusalem Artichoke by up-regulation of antioxidant enzyme. Pedosphere 18:766–774
Yahubyan G, Gozmanova M, Denev I, Toneva V, Minkov I (2009) Prompt response of superoxide dismutase and peroxidase to dehydration and rehydration of the resurrection plant Haberlearhodopensis. Plant Growth Regul 57:49–56
Yamada M, Morishita H, Urano K, Shiozaki N, Yamaguchi-Shinozaki K, Shinozaki K, Yoshiba Y (2005) Effects of free proline accumulation in petunias under drought stress. J Exp Bot 56:1975–1981
Yamato M, Ikeda S, Iwase K (2008) Community of arbuscular mycorrhizal fungi in a coastal vegetation on Okinawa Island and effect of the isolated fungi on growth of sorghum under salt-treated conditions. Mycorrhiza 18:241–249
Yemm EW, Willis AJ (1954) The respiration of barley plants. IX. The metabolism of roots during assimilation of nitrogen. New Phytotol 55:229–234
Yooyongwech S, Phaukinsang N, Cha-Um S, Supaibulwatana K (2013) Arbuscula rmycorrhiza improved growth performance in Macadamia tetraphylla L. grown under water deficit stress involves soluble sugar and proline accumulation. Plant Growth Regul 69:285–293. https://doi.org/10.1007/s10725-012-9771-6
Zhang F, Zou YN, Wu QS, Kuča K (2020) Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environ Exp Bot 171:103926
Zhao C, Zhang H, Song C, Zhu J, Shabala Z (2020) Mechanisms of plant responses and adaptation to soil salinity. Innovation 1:100017
Zheng C, Jiang D, Liu F, Dai T, Liu W, Jing Q, Cao W (2009) Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ Exp Bot 67:222–227
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167:527–533
Zou P, Li K, Liu S, He X, Zhang X et al (2016) Effect of sulfated chitooligosaccharides on wheat seedlings (Triticum aestivumL.) under salt stress. J Agric Food Chem 64:2815–2821. https://doi.org/10.1021/acs.jafc.5b05624
Funding
The authors declare that no funding is applicable
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.S. Sadak and M.G. Dawood declare that they have no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sadak, M.S., Dawood, M.G. Biofertilizer Role in Alleviating the Deleterious Effects of Salinity on Wheat Growth and Productivity. Gesunde Pflanzen 75, 1207–1219 (2023). https://doi.org/10.1007/s10343-022-00783-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-022-00783-3