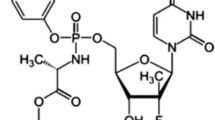
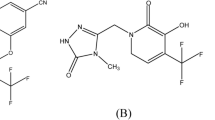
Abstract
Elvitegravir (ETV), drug substance, and its eleven process-related impurities have been identified and their structural identification study has been carried out with the aid of 1H, 13C NMR, and ESI–LC–MS spectroscopic techniques. Plausible fragments were also proposed for each impurity to ascertain its structure. Simple, facile, and selective, stability indicating, mass spectrometry compatible HPLC method has been developed and subsequently validated with the validation parameters of specificity, LOD, LOQ, precision at LOQ, linearity, accuracy at LOQ to 120% levels, method precision, intermediate precision studies, and solution stability has also been established. This method encompasses a simple gradient mode of separation with mobile phases—(A) 0.1% trifluoroacetic acid in water and (B) 0.1% trifluoroacetic acid in acetonitrile, the mass sectrometric compatible mobile phase has been chosen for the identification of known, unknown and degradation impurities. To assess the nature of each impurity, whether they are either process-related or degradation-induced, an intensive stress study has also been conducted. From this degradation assessment, all the impurities have been classified as process-related. Further, the assessment of three different manufacturers samples was also executed to show the method applicability and comparison of quality of the different manufacturers drug, and thus this method shall be engaged as a quality inferring tool for the marketed sample.















Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding on reasonable request.
References
Prada N, Markowitz M (2010) Novel integrase inhibitors for HIV. Expert Opin Investig Drugs 19:1087–1098. https://doi.org/10.1517/13543784.2010.501078
Siberry GK, Hazra R (2018) 113-Management of HIV infection. In: Long SS, Fischer M, Prober CG (eds) principles and practice of pediatric infectious diseases, 5 edn. Elsevier, Amsterdam, p 681.e2. https://doi.org/10.1016/B978-0-323-40181-4.00113-4
Eckhardt BJ, Gulick RM (2017) 152—drugs for HIV infection. In Cohen J, Powderly WG, Opal SM (eds) Infectious diseases, 4 edn. Elsevier, Amsterdam, pp 1293–1308. e2. https://doi.org/10.1016/B978-0-7020-6285-8.00152-0
Tsibris AMN, Hirsch MS (2015) 130—antiretroviral therapy for human immuno deficiency virus infection. In: Bennett JE, Dolin R, Blaser MJ, Mandell, Douglas (eds) Principles and practice of infectious diseases, 8 edn. WB Saunders, Elsevier, Amsterdam, pp 1622–1641. e6. https://doi.org/10.1016/B978-1-4557-4801-3.00130-2
Waller DG, Sampson AP (2018) 51—chemotherapy of infections. In: Waller DG, Sampson AP (eds) Medical pharmacology and therapeutics, 5 edn. Elsevier, Amsterdam, p 581. https://doi.org/10.1016/B978-0-7020-7167-6.00051-8
Brody T (2018) Chapter 4—dose modification and dose titration. In: Brody T (ed) FDA’s Drug review process and the package label. Academic Press, Camebridge, p 101. https://doi.org/10.1016/B978-0-12-814647-7.00004-X
Isobel DR, Jodi ML, Catherine PO, Ana LGC, Li HK, Barker CIS (2014) Chapter 29—antiviral drugs. In: Ray SD (ed) Side effects of drugs annual. Elsevier, Amsterdam, pp 36: 401. https://doi.org/10.1016/B978-0-444-63407-8.00029-0
Brehm TT, Franz M, Hüfner A, Hertling S, Schmiedel S, Degen O, Kreuels B, Wiesch JSZ (2019) Safety and efficacy of elvitegravir, dolutegravir, and raltegravir in a real-world cohort of treatment-naïve and -experienced patients. Medicine (Baltimore) 98:e16721. https://doi.org/10.1097/MD.0000000000016721
Gong Y, Chowdhury P, Nagesh PKB, Rahman MA, Zhi K, Yallapu MM, Kumar S (2020) Novel elvitegravir nanoformulation for drug delivery across the blood-brain barrier to achieve HIV-1 suppression in the CNS macrophages. Sci Rep 10:3835. https://doi.org/10.1038/s41598-020-60684-1
Bruce RD, Katz EM, Kharasch ED, Moody DE, Morse GD (2006) Pharmacokinetic interactions between buprenorphine and antiretroviral medications. Clin Infect Dis 43:S216. https://doi.org/10.1086/508186
Satpathy R, Ghosh S (2011) In-silico comparative study and quantitative structure-activity relationship analysis of some structural and physiochemical descriptors of elvitegravir analogs. J Young Pharm 3:246. https://doi.org/10.4103/0975-1483.83776
Unger NR, Worley MV, Kisgen JJ, Sherman EM, Childs-Kean LM (2016) Elvitegravir for the treatment of HIV. Expert Opin Pharmacother 17:2359. https://doi.org/10.1080/14656566.2016.1250885
Momper JD, Best BM, Wang J, Capparelli EV, Stek A, Barr E, Badell ML, Acosta EP, Purswani M, Smith E, Chakhtoura N, Park K, Burchett S, Shapiro DE, Mirochnick M (2018) IMPAACT P1026s protocol team. Elvitegravir/cobicistat pharmacokinetics in pregnant and postpartum women with HIV. AIDS 32:2507. https://doi.org/10.1097/QAD.0000000000001992
(2016) Elvitegravir (Vitekta) for HIV Med Lett Drugs Ther 5:10–1. PMID: 26761343
Prathipati PK, Mandal S, Destache C (2018) LC-MS/MS method for the simultaneous determination of tenofovir, emtricitabine, elvitegravir and rilpivirine in dried blood spots. J Biomed Chromatogr 26:e4270. https://doi.org/10.1002/bmc.4270
Penchala SD, Fawcett S, Else L, Egan D, Amara A, Elliot E, Challenger E, Back D, Boffito M, Khoo S (2016) The development and application of a novel LC-MS/MS method for the measurement of Dolutegravir, Elvitegravir and Cobicistat in human plasma. J Chromatogr B Anal Technol Biomed Life Sci 1027:174. https://doi.org/10.1016/j.jchromb.2016.05.040
Ocque AJ, Hagler CE, Morse GD, Letendre SL, Ma Q (2018) Development and validation of an LC-MS/MS assay for tenofovir and tenofovir alafenamide in human plasma and cerebrospinal fluid. J Pharm Biomed Anal 156:163. https://doi.org/10.1016/j.jpba.2018.04.035
Simiele M, Ariaudo A, De Nicolò A, Favata F, Ferrante M, Carcieri C, Bonora S, Di Perri G, De Avolio A (2017) UPLC-MS/MS method for the simultaneous quantification of three new antiretroviral drugs, dolutegravir, elvitegravir and rilpivirine, and other thirteen antiretroviral agents plus cobicistat and ritonavir boosters in human plasma. J Pharm Biomed Anal 138:223. https://doi.org/10.1016/j.jpba.2017.02.002
Bollen PDJ, De Graaff-Teulen MJA, Schalkwijk S, Van Erp NP, Burger DM (2019) Development and validation of an UPLC-MS/MS bioanalytical method for simultaneous quantification of the antiretroviral drugs dolutegravir, elvitegravir, raltegravir, nevirapine and etravirine in human plasma. J Chromatogr B Anal Technol Biomed Life Sci 1105:76. https://doi.org/10.1016/j.jchromb.2018.12.008
Djerada Z, FeliuC TC, Vautier D, Binet L, Robinet A, Marty H, Gozalo C, Lamiable D, Millart H (2013) Validation of a fast method for quantitative analysis of elvitegravir, raltegravir, maraviroc, etravirine, tenofovir, boceprevir and 10 other antiretroviral agents in human plasma samples with a new UPLC-MS/MS technology. J Pharm Biomed Anal 86:100. https://doi.org/10.1016/j.jpba.2013.08.002
Sengupta P,Chatterjee B,Tekade RK (2018) Current regulatory requirements and practical approaches for stability analysis of pharmaceutical products: a comprehensive review. Int J Pharm 543:328. https://doi.org/10.1016/j.ijpharm.2018.04.007
Yanagisawa K (2015) Transition of psychotropic drugs in japanese pharmacopoeia (JP) (Part 15). Transitions in the standards and test methods of potassium bromide in JP I (1886) and JP X VI (2011), and Comparison between the USP and BP. Yakushigaku Zasshi 50:13
Kameyama Y, Matsuhama M, Mizumaru C, Saito R, Ando T, Miyazaki S (2019) Comparative study of pharmacopoeias in Japan, Europe, and the United States: toward the further convergence of international pharmacopoeial standards. Chem Pharm Bull (Tokyo) 67:1301. https://doi.org/10.1248/cpb.c19-00621
Reddy GM, Bhaskar BV, Reddy PP, Ashok S, Sudhakar P, Babu JM, Vyas K, Mukkanti K (2007) Structural identification and characterization of potential impurities of pantoprazole sodium. J Pharm Biomed Anal 45:201–210. https://doi.org/10.1016/j.jpba.2007.05.032
Bharathi Ch, Prasad ChS, Bharathi DV, Shankar R, Rao VJ, Dandala R, Naidu A (2007) Structural identification and characterization of impurities in ceftizoxime sodium. J Pharm Biomed Anal 43:733–740. https://doi.org/10.1016/j.jpba.2006.07.031
Maggio RM, Calvo NL, Vignaduzzo SE, Kaufman TS (2014) Pharmaceutical impurities and degradation products: uses and applications of NMR techniques. J Pharma Biomed Anal 101:102–122. https://doi.org/10.1016/j.jpba.2014.04.016
Thomas S, Paul SK, Shandilya S, Agarwal A, Saxena N, Awasthi AK, Matta HB, Vir D, Mathela CS (2012) Identification and structural elucidation of two process impurities and stress degradants in darifenacin hydrobromide active pharmaceutical ingredient by LC-ESI/MSn. Analyst 137:3571–3582. https://doi.org/10.1039/C2AN35454C
De Alvarenga Junior BR, Carneiro RL (2019) Chemometrics approaches in forced degradation studies of pharmaceutical drugs. Molecules 24:3804. https://doi.org/10.3390/molecules24203804
Shankar G, Borkar RM, Suresh U, Guntuku L, Naidu VGM, Nagesh N, Srinivas R (2017) Forced degradation studies of lansoprazole using LC-ESI HRMS and 1H-NMR experiments: in vitro toxicity evaluation of major degradation products. J Mass Spectrom 52:459–471. https://doi.org/10.1002/jms.3949
Sambandan E, Kathavarayan T, Sellappan S, Shiea J, Ponnusamy VK (2019) Identification and characterization of unknown degradation impurities in beclomethasone dipropionate cream formulation using HPLC, ESI-MS and NMR. J Pharm Biomed Anal 167:123–131. https://doi.org/10.1016/j.jpba.2019.02.013
Zhao L, Wang Q, Bie Y, Lu X (2017) Isolation, identification and characterization of potential impurities of anidulafungin. J Pharm Biomed Anal 141:192–199. https://doi.org/10.1016/j.jpba.2017.04.014
Sahu PK, Ramisetti NR, Cecchi T,Swain S,Patro CS, Panda J (2018) An overview of experimental designs in HPLC method development and validation. J Pharm Biomed Anal 147: 590–611. https://doi.org/10.1016/j.jpba.2017.05.006
Arumugam A, Joshi A, Vasu KK (2017) Development and validation of a stability-indicating HPLC method for imidapril and its degradation products using a design of experiment (DoE) approach. J AOAC Int 100:1727–1738. https://doi.org/10.5740/jaoacint.16-0329
Vitekta EMA assessment report, EMA/701401/2013 (2013) https://www.ema.europa.eu/en/documents/assessment-report/vitekta-epar-public-assessment-report_en.pdf
ICH Q2 (R1), Validation of analytical procedures: text and methodology, 1995. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2-r1-validation-analytical-procedures-text-and-methodology/. Accessed 20 April 2020
Patel VB, Patel AD, Shah DA (2018) Stability indicating liquid chromatographic method for simultaneous determination of aspirin and omeprazole. Curr Drug DiscovTechnol 15:351–360. https://doi.org/10.2174/1570163814666171023144105
Saini S, Sharma T, Patel A, Kaur R, Tripathi SK, Katare OP, Singh B (2020) QbD-steered development and validation of an RP-HPLC method for quantification of ferulic acid: rational application of chemometric tools. J Chromatogr B: Analyt Technol Biomed Life Sci 1155:122300. https://doi.org/10.1016/j.jchromb.2020.122300
Wiberg K, Andersson M, Hagman A, Jacobsson SP (2004) Peak purity determination with principal component analysis of high-performance liquid chromatography-diode array detection data. J Chromatogr A 1029:13–20. https://doi.org/10.1016/j.chroma.2003.12.052
Nikolin B, Imamović B, Medanhodzić-Vuk S, Sober M (2004) High perfomance liquid chromatography in pharmaceutical analyses. Bosn J Basic Med Sci 4:5–9. https://doi.org/10.17305/bjbms.2004.3405
Author information
Authors and Affiliations
Contributions
Elumalai performed the experimental work, Sellappan, Mamidala, NIshtala, and Thenmozhi wrote the main manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elumalai, S., Senthilkumar, S., Srikanth, M. et al. Characterization of Elvitegravir and Its Related Impurities Using ESI–LC–MS, NMR Techniques, Method Development and Validation of Its Related Substances by HPLC Method. Chromatographia 87, 227–248 (2024). https://doi.org/10.1007/s10337-024-04314-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-024-04314-2