Abstract
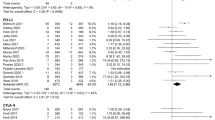
The introduction of immune checkpoint inhibitors (ICIs) has reshaped the therapy of hepatocellular carcinoma (HCC). ICIs are a novel therapy with frequent adverse events (AEs), including treatment-related adverse events (trAEs) and immune-related adverse events (irAEs). However, no comprehensive overview of the toxicity spectrum of ICIs in HCC patients has been provided. Electronic databases were searched to identify eligible studies. A meta-analysis of the incidence rate of AEs in HCC patients treated with ICIs was performed. Lastly, the prognostic value of irAEs in HCC patients treated with ICIs was verified. Forty-seven studies with 6472 participations met the inclusion criteria. The pooled all-grade trAEs incidence rate was 83.4% (95% confidence interval [95% CI] 77.0–89.1%), ≥ grade 3 trAEs incidence rate was 33.0% (95% CI 26.9–39.5%), all-grade irAEs incidence rate was 34% (95% CI 22–47%), and ≥ grade 3 irAEs incidence rate was 9% (95% CI 5–14%). Aspartate aminotransferase (AST) increase (38%, 95% CI 35–40%) is the most common trAEs. Fatigue (14%, 95% CI 7–23%) is the most common irAEs. The pooled results also showed that 18.8% (95% CI 13.2–25.2%) of patients required systemic steroid therapy due to AEs, while 6.6% (95% CI 4.6–9.0%) of patients withdrew from treatment due to AEs. Additionally, patients experiencing irAEs may have a better progression-free survival (PFS) (multivariate analysis: hazard ratio [HR] = 0.41, 95% CI 0.27–0.61, I2 = 36.3%) but not overall survival (OS) (multivariate analysis: HR = 0.54, 95% CI 0.22–1.36, I2 = 83.2%) than those with no irAEs. Our study presents a systemic assessment of the AEs profile in HCC patients receiving ICIs, providing important reference for clinicians on toxicity profile. Besides, patients with irAEs may have a better PFS. More large-scale and prospective studies are needed to confirm our conclusions.





Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- ICIs:
-
Immune checkpoint inhibitors
- HCC:
-
Hepatocellular carcinoma
- AEs:
-
Adverse events
- trAEs:
-
Treatment-related adverse events
- irAEs:
-
Immune-related adverse events
- 95% CI:
-
95% Confidence interval
- HR:
-
Hazard ratio
- AST:
-
Aspartate aminotransferase
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- aHCC:
-
Advanced hepatocellular carcinoma
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death ligand-1
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein-4
- VEGF:
-
Vascular endothelial growth factor
- NSCLC:
-
Non-small cell lung carcinoma
- RCT:
-
Randomized control trial
- ORR:
-
Objective response rate
- DCR:
-
Disease control rate
- RECIST:
-
Response evaluation criteria in solid tumors
- mRECIST:
-
Modified response evaluation criteria in solid tumors
- CTCAE:
-
Common terminology criteria for adverse events
- ALT:
-
Alanine aminotransferase
- ECOG:
-
Eastern Cooperative Oncology Group
- Tregs:
-
Regulatory T cells
- Th1:
-
CD4 + helper T cells 1
- IL-6/17:
-
Interleukin-6/17
- Th-17:
-
T helper cell 17
- HPD:
-
Hyperprogressive disease
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Verset G, Borbath I, Karwal M, et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: data from the open-label, phase II KEYNOTE-224 trial. Clin Cancer Res. 2022;28:2547–54. https://doi.org/10.1158/1078-0432.CCR-21-3807.
Zhou M, Liu B, Shen J. Immunotherapy for hepatocellular carcinoma. Clin Exp Med. 2022. https://doi.org/10.1007/s10238-022-00874-5.
Fessas P, Kaseb A, Wang Y, et al. Post-registration experience of nivolumab in advanced hepatocellular carcinoma: an international study. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2020-001033.
Rizzo A, Ricci AD, Gadaleta-Caldarola G, Brandi G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: current management and future challenges. Expert Rev Gastroenterol Hepatol. 2021;15:1245–51. https://doi.org/10.1080/17474124.2021.1973431.
Rizzo A, Ricci AD, Di Federico A, Frega G, Palloni A, Tavolari S, Brandi G. Predictive biomarkers for checkpoint inhibitor-based immunotherapy in hepatocellular carcinoma: Where do we stand? Front Oncol. 2021;11: 803133. https://doi.org/10.3389/fonc.2021.803133.
Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38:4317–45. https://doi.org/10.1200/jco.20.02672.
Liu HT, Jiang MJ, Deng ZJ, Li L, Huang JL, Liu ZX, Li LQ, Zhong JH. Immune checkpoint inhibitors in hepatocellular carcinoma: current progresses and challenges. Front Oncol. 2021;11: 737497. https://doi.org/10.3389/fonc.2021.737497.
Sangro B, Chan SL, Meyer T, Reig M, El-Khoueiry A, Galle PR. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol. 2020;72:320–41. https://doi.org/10.1016/j.jhep.2019.10.021.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68. https://doi.org/10.1056/NEJMra1703481.
De Velasco G, Je Y, Bosse D, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5:312–8. https://doi.org/10.1158/2326-6066.CIR-16-0237.
Kfoury M, Najean M, Lappara A, et al. Analysis of the association between prospectively collected immune-related adverse events and survival in patients with solid tumor treated with immune-checkpoint blockers, taking into account immortal-time bias. Cancer Treat Rev. 2022;110: 102452. https://doi.org/10.1016/j.ctrv.2022.102452.
Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF. Preferred reporting items for a systematic review and meta-analysis of individual participant data. JAMA. 2015. https://doi.org/10.1001/jama.2015.3656.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. https://doi.org/10.1007/s10654-010-9491-z.
Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898. https://doi.org/10.1136/bmj.l4898.
Wu Z, Chen Q, Qu L, et al. Adverse events of immune checkpoint inhibitors therapy for urologic cancer patients in clinical trials: a collaborative systematic review and meta-analysis. Eur Urol. 2022;81:414–25. https://doi.org/10.1016/j.eururo.2022.01.028.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. Anz J Surg. 2003;73:712–6. https://doi.org/10.1046/j.1445-2197.2003.02748.x.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. https://doi.org/10.1186/2049-3258-72-39.
Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–73. https://doi.org/10.1016/j.jhep.2021.11.030.
D’Alessio A, Fulgenzi CAM, Nishida N, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology. 2022. https://doi.org/10.1002/hep.32468.
El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (No Pagination). 2017. https://doi.org/10.1016/S0140-6736%2817%2931046-2.
Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–70. https://doi.org/10.1200/jco.20.00808.
Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. https://doi.org/10.1200/JCO.19.01307.
Han C, Ye S, Hu C, et al. Clinical activity and safety of penpulimab (anti-PD-1) with anlotinib as first-line therapy for unresectable hepatocellular carcinoma: an open-label, multicenter, phase Ib/II trial (AK105-203). Front Oncol. 2021;11: 684867. https://doi.org/10.3389/fonc.2021.684867.
Kelley RK, Sangro B, Harris W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol: Offic J Am Soc Clin Oncol. 2021;39:2991–3001. https://doi.org/10.1200/JCO.20.03555.
Kudo M, Matilla A, Santoro A, et al. CheckMate 040 cohort 5: a phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol. 2021;75:600–9. https://doi.org/10.1016/j.jhep.2021.04.047.
Kuo YH, Yen YH, Chen YY, Kee KM, Hung CH, Lu SN, Hu TH, Chen CH, Wang JH. Nivolumab versus regorafenib in patients with hepatocellular carcinoma after sorafenib failure. Front Oncol. 2021;11: 683341. https://doi.org/10.3389/fonc.2021.683341.
Lee MS, Ryoo BY, Hsu CH, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808–20. https://doi.org/10.1016/S1470-2045(20)30156-X.
Li H, Qin S, Liu Y, et al. Camrelizumab combined with FOLFOX4 regimen as first-line therapy for advanced hepatocellular carcinomas: a sub-cohort of a multicenter phase Ib/II study. Drug Des Devel Ther. 2021;15:1873–82. https://doi.org/10.2147/dddt.S304857.
Monge C, Xie C, Steinberg SM, Greten TF. Clinical indicators for long-term survival with immune checkpoint therapy in advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:507–12. https://doi.org/10.2147/jhc.S311496.
Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–80. https://doi.org/10.1016/S1470-2045(20)30011-5.
Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22:977–90. https://doi.org/10.1016/S1470-2045(21)00252-7.
Tai D, Loke K, Gogna A, et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209–678): a single arm, single centre, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:1025–35. https://doi.org/10.1016/S2468-1253(21)00305-8.
Teng Y, Ding X, Li W, Sun W, Chen J. A retrospective study on therapeutic efficacy of transarterial chemoembolization combined with immune checkpoint inhibitors plus lenvatinib in patients with unresectable hepatocellular carcinoma. Technol Cancer Res Treat. 2022;21:15330338221075174. https://doi.org/10.1177/15330338221075174.
Wu JY, Yin ZY, Bai YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–40. https://doi.org/10.2147/jhc.S332420.
Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27:1003–11. https://doi.org/10.1158/1078-0432.Ccr-20-2571.
Yang F, Yang J, Xiang W, et al. Safety and efficacy of transarterial chemoembolization combined with immune checkpoint inhibitors and tyrosine kinase inhibitors for hepatocellular carcinoma. Front Oncol. 2021;11: 657512. https://doi.org/10.3389/fonc.2021.657512.
Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6: e204564. https://doi.org/10.1001/jamaoncol.2020.4564.
Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. https://doi.org/10.1016/S1470-2045(21)00604-5.
Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–52. https://doi.org/10.1016/S1470-2045(18)30351-6.
Chon YE, Cheon J, Kim H, Kang B, Ha Y, Kim DY, Hwang SG, Chon HJ, Kim BK. Predictive biomarkers of survival in patients with advanced hepatocellular carcinoma receiving atezolizumab plus bevacizumab treatment. Cancer Med. 2022. https://doi.org/10.1002/cam4.5161.
Chuma M, Uojima H, Hattori N, et al. Safety and efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma in early clinical practice: a multicenter analysis. Hepatol Res. 2022;52:269–80. https://doi.org/10.1111/hepr.13732.
Himmelsbach V, Pinter M, Scheiner B, et al. Efficacy and Safety of Atezolizumab and Bevacizumab in the Real-World Treatment of Advanced Hepatocellular Carcinoma: Experience from Four Tertiary Centers. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14071722.
Huang J, Guo Y, Huang W, et al. Regorafenib combined with PD-1 blockade immunotherapy versus regorafenib as second-line treatment for advanced hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2022;9:157–70. https://doi.org/10.2147/jhc.S353956.
Iwamoto H, Shimose S, Noda Y, et al. Initial experience of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in real-world clinical practice. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13112786.
Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995–1008. https://doi.org/10.1016/s1470-2045(22)00326-6.
Kim BK, Cheon J, Kim H, Kang B, Ha Y, Kim DY, Hwang SG, Chon YE, Chon HJ (2022) Atezolizumab/bevacizumab vs. lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a real-world, multi-center study. Cancers (Basel). https://doi.org/10.3390/cancers14071747
Lai Z, He M, Bu X, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68–77. https://doi.org/10.1016/j.ejca.2022.07.005.
Maesaka K, Sakamori R, Yamada R, et al. Comparison of atezolizumab plus bevacizumab and lenvatinib in terms of efficacy and safety as primary systemic chemotherapy for hepatocellular carcinoma. Hepatol Res. 2022;52:630–40. https://doi.org/10.1111/hepr.13771.
Ng KYY, Wong LWJ, Ang AJS, Tan SH, Choo SP, Tai DW, Lee JJX. Real-world efficacy and safety of immune checkpoint inhibitors in advanced hepatocellular carcinoma: experience of a tertiary Asian Center. Asia Pac J Clin Oncol. 2021;17:e249–61. https://doi.org/10.1111/ajco.13454.
Vithayathil M, D’Alessio A, Fulgenzi CAM, et al. Impact of older age in patients receiving atezolizumab and bevacizumab for hepatocellular carcinoma. Liver Int. 2022. https://doi.org/10.1111/liv.15405.
Wang JH, Chen YY, Kee KM, et al. The prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma receiving atezolizumab plus bevacizumab. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14020343.
Xin Y, Cao F, Yang H, Zhang X, Chen Y, Cao X, Zhou X, Li X, Zhou J. Efficacy and safety of atezolizumab plus bevacizumab combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Front Immunol. 2022;13: 929141. https://doi.org/10.3389/fimmu.2022.929141.
Yao J, Zhu X, Wu Z, et al. Efficacy and safety of PD-1 inhibitor combined with antiangiogenic therapy for unresectable hepatocellular carcinoma: a multicenter retrospective study. Cancer Med. 2022. https://doi.org/10.1002/cam4.4747.
Zhang JX, Chen YX, Zhou CG, Liu J, Liu S, Shi HB, Zu QQ. Efficacy and safety of the combination of transarterial chemoembolization with camrelizumab plus apatinib for advanced hepatocellular carcinoma: a retrospective study of 38 patients from a single center. Can J Gastroenterol Hepatol. 2022;2022:7982118. https://doi.org/10.1155/2022/7982118.
Zhao L, Chang N, Shi L, Li F, Meng F, Xie X, Xu Z, Wang F. Lenvatinib plus sintilimab versus lenvatinib monotherapy as first-line treatment for advanced HBV-related hepatocellular carcinoma: a retrospective, real-world study. Heliyon. 2022;8: e09538. https://doi.org/10.1016/j.heliyon.2022.e09538.
Zou J, Huang P, Ge N, Xu X, Wang Y, Zhang L, Chen Y. Anti-PD-1 antibodies plus lenvatinib in patients with unresectable hepatocellular carcinoma who progressed on lenvatinib: a retrospective cohort study of real-world patients. J Gastrointest Oncol. 2022;13:1898–906. https://doi.org/10.21037/jgo-22-643.
Hsu WF, Chuang PH, Chen CK, et al. Predictors of response and survival in patients with unresectable hepatocellular carcinoma treated with nivolumab: real-world experience. Am J Cancer Res. 2020;10:4547–60.
Lu L, Xing K, Wei W, et al. Immune-related adverse events predict responses to PD-1 blockade immunotherapy in hepatocellular carcinoma. Int J Cancer. 2021. https://doi.org/10.1002/ijc.33609.
Ng KYY, Tan SH, Tan JJE, et al. Impact of immune-related adverse events on efficacy of immune checkpoint inhibitors in patients with advanced hepatocellular carcinoma. Liver Cancer. 2022;11:9–21. https://doi.org/10.1159/000518619.
Pinato DJ, Marron TU, Mishra-Kalyani PS, et al. Treatment-related toxicity and improved outcome from immunotherapy in hepatocellular cancer: evidence from an FDA pooled analysis of landmark clinical trials with validation from routine practice. Eur J Cancer. 2021;157:140–52. https://doi.org/10.1016/j.ejca.2021.08.020.
Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. 2020;13:47. https://doi.org/10.1186/s13045-020-00886-2.
Xu S, Lai R, Zhao Q, Zhao P, Zhao R, Guo Z. Correlation between immune-related adverse events and prognosis in hepatocellular carcinoma patients treated with immune checkpoint inhibitors. Front Immunol. 2021;12: 794099. https://doi.org/10.3389/fimmu.2021.794099.
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. https://doi.org/10.1038/s41572-020-00240-3.
Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345–62. https://doi.org/10.1016/s0140-6736(22)01200-4.
D’Alessio A, Fulgenzi CAM, Nishida N, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology. 2022;76:1000–12. https://doi.org/10.1002/hep.32468.
Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5:1008–19. https://doi.org/10.1001/jamaoncol.2019.0393.
Peeraphatdit TB, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity from immune checkpoint inhibitors: a systematic review and management recommendation. Hepatology. 2020;72:315–29. https://doi.org/10.1002/hep.31227.
Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind. Phase III trial. J Clin Oncol. 2020;38:193–202. https://doi.org/10.1200/JCO.19.01307.
Cui TM, Liu Y, Wang JB, Liu LX. Adverse effects of immune-checkpoint inhibitors in hepatocellular carcinoma. Onco Targets Ther. 2020;13:11725–40. https://doi.org/10.2147/OTT.S279858.
Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6:865–71. https://doi.org/10.1001/jamaoncol.2020.0726.
Robert C, Hwu W-J, Hamid O, et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: a landmark analysis in patients with advanced melanoma. Eur J Cancer. 2021;144:182–91. https://doi.org/10.1016/j.ejca.2020.11.010.
Xu Y, Fu Y, Zhu B, Wang J, Zhang B. Predictive biomarkers of immune checkpoint inhibitors-related toxicities. Front Immunol. 2020;11:2023. https://doi.org/10.3389/fimmu.2020.02023.
Goswami S, Siddiqui BA, Subudhi SK, Basu S, Yadav SS, Diab A, Sharma P. A composite T cell biomarker in pre-treatment blood samples correlates with detection of immune-related adverse events. Cancer Cell. 2022;40:249–51. https://doi.org/10.1016/j.ccell.2022.02.015.
Hailemichael Y, Johnson DH, Abdel-Wahab N, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell. 2022;40(509–23): e6. https://doi.org/10.1016/j.ccell.2022.04.004.
Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543–52. https://doi.org/10.1001/jamaoncol.2018.3676.
Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020;18:87. https://doi.org/10.1186/s12916-020-01549-2.
Fujii T, Colen RR, Bilen MA, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs. 2018;36:638–46. https://doi.org/10.1007/s10637-017-0534-0.
Chen J, Zhang D, Yuan Y. Anti-PD-1/PD-L1 immunotherapy in conversion treatment of locally advanced hepatocellular carcinoma. Clin Exp Med. 2022. https://doi.org/10.1007/s10238-022-00873-6.
Funding
This work was supported by the grants from the Taishan Scholars Program of Shandong Province, National Natural Science Foundation of China (Grant Nos. 82073200, 81874178), major basic research of Shandong Provincial Natural Science Foundation (Grant No. ZR202105070027), and funds for Independent Cultivation of Innovative Team from Universities in Jinan (Grant No. 2020GXRC023).
Author information
Authors and Affiliations
Contributions
JCT, HL, and TL designed the study; JCT, LJY, and ZND contributed to data search from databases; HL, LJY, and ZND formulated inclusion criteria; JCT, HL, JSX, XCM, and YCY selected eligible studies; JCT, CLH, BWT, SYT, ZRD, DXW, XCM, and YCY acquired, analyzed, or interpreted data; JCT and HL assessed study quality and wrote the manuscript; TL resolved differences and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jin-Cheng Tian and Hui Liu have contributed equally to this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, JC., Liu, H., Yan, LJ. et al. Adverse events of immune checkpoint inhibitors in hepatocellular carcinoma: a systemic review and meta-analysis. Clin Exp Med 23, 2115–2129 (2023). https://doi.org/10.1007/s10238-022-00938-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00938-6