Abstract
Objective
The EORTC QLU-C10D is a new preference-based measure derived from the EORTC QLQ-C30. Country-specific value sets are required to support the cost-utility analysis of cancer-related interventions. This study aimed to generate an EORTC QLU-C10 value set for Hong Kong (HK).
Methods
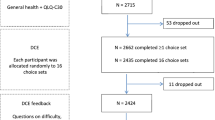
A HK online panel was quota-sampled to achieve an adult general population sample representative by sex and age. Participants were invited to complete an online discrete choice experiment survey. Each participant was asked to complete 16 choice-pairs, randomly assigned from a total of 960 choice-pairs, each comprising two QLU-C10D health states and a duration attribute. Conditional and mixed logistic regression analyses were used to analyse the data.
Results
The analysis included data from 1041 respondents who had successfully completed the online survey. The distribution of sex did not differ from that of the general population, but a significant difference was found among age groups. A weighting analysis for non-representative variable (age) was used. Utility decrements were generally monotonic, with the largest decrements for physical functioning (− 0.308), role functioning (− 0.165), and pain (− 0.161). The mean QLU-C10D utility score of the participants was 0.804 (median = 0.838, worst to best = − 0.169 to 1). The value of the worst health state was − 0.223, which was sufficiently lower than 0 (being dead).
Conclusions
This study established HK utility weights for the QLU-C10D, which can facilitate cost-utility analyses across cancer-related health programmes and technologies.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Brazier, J.E., Rowen, D., Lloyd, A., et al.: Future directions in valuing benefits for estimating QALYs: is time up for the EQ-5D? Value Health 22, 62–68 (2019)
Norman, R., Mercieca-Bebber, R., Rowen, D., et al.: UK utility weights for the EORTC QLU-C10D. Health Econ. 28, 1385–1401 (2019). https://doi.org/10.1002/hec.3950
Herdman, M., Gudex, C., Lloyd, A., et al.: Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 20, 1727–1736 (2011). https://doi.org/10.1007/s11136-011-9903-x
Brazier, J., Roberts, J., Deverill, M.: The estimation of a preference-based measure of health from the SF-36. J. Health Econ. 21, 271–292 (2002). https://doi.org/10.1016/S0167-6296(01)00130-8
Versteegh, M.M., Leunis, A., Uyl-de Groot, C.A., et al.: Condition-specific preference-based measures: benefit or burden? Value Health 15, 504–513 (2012). https://doi.org/10.1016/j.jval.2011.12.003
Rowen, D., Brazier, J., Ara, R., et al.: The role of condition-specific preference-based measures in health technology assessment. Pharmacoeconomics 35, 33–41 (2017). https://doi.org/10.1007/s40273-017-0546-9
Giesinger, J.M., Efficace, F., Aaronson, N., et al.: Past and current practice of patient-reported outcome measurement in randomized cancer clinical trials: a systematic review. Value Health 24, 585–591 (2021). https://doi.org/10.1016/j.jval.2020.11.004
McTaggart-Cowan, H., King, M.T., Norman, R., et al.: The EORTC QLU-C10D: the Canadian Valuation Study and algorithm to derive cancer-specific utilities from the EORTC QLQ-C30. MDM Policy Pract 4, 238146831984253 (2019). https://doi.org/10.1177/2381468319842532
Revicki, D.A., King, M.T., Viney, R., et al.: United states utility algorithm for the EORTC QLU-C10D, a multiattribute utility instrument based on a cancer-specific quality-of-life instrument. Med. Decis. Mak. 41, 485–501 (2021). https://doi.org/10.1177/0272989X211003569
King, M.T., Viney, R., Simon Pickard, A., et al.: Australian utility weights for the EORTC QLU-C10D, a multi-attribute utility instrument derived from the cancer-specific quality of life questionnaire, EORTC QLQ-C30. Pharmacoeconomics 36, 225–238 (2018). https://doi.org/10.1007/s40273-017-0582-5
Kemmler, G., Gamper, E., Nerich, V., et al.: German value sets for the EORTC QLU-C10D, a cancer-specific utility instrument based on the EORTC QLQ-C30. Qual. Life Res. 28, 3197–3211 (2019). https://doi.org/10.1007/s11136-019-02283-w
Nerich, V., Gamper, E.M., Norman, R., et al.: French value-set of the QLU-C10D, a cancer-specific utility measure derived from the QLQ-C30. Appl. Health Econ. Health Policy (2020). https://doi.org/10.1007/s40258-020-00598-1
Gamper, E.M., King, M.T., Norman, R., et al.: EORTC QLU-C10D value sets for Austria, Italy, and Poland. Qual. Life Res. 29, 2485–2495 (2020). https://doi.org/10.1007/s11136-020-02536-z
Atlas TC. Southern, Eastern, & South-Eastern Asia. https://canceratlas.cancer.org/the-burden/south-east-se-asia/. Accessed 28 May 2021
Hong Kong Cancer Registry. Overview of Hong Kong Cancer Statistics. Hong Kong Hospital Authority (2020). https://www3.ha.org.hk/cancereg. Accessed 28 May 2021
Campolina, A.G., Yuba, T.Y., Decimoni, T.C., et al.: Health economic evaluations of cancer in Brazil: a systematic review. Front. Public Health 6, 205 (2018). https://doi.org/10.3389/fpubh.2018.00205
Johnson, J.A., Luo, N., Shaw, J.W., et al.: Valuations of EQ-5D health states: are the United States and United Kingdom different? Med. Care 43, 221–228 (2005). https://doi.org/10.1097/00005650-200503000-00004
Zhao, H., Kanda, K.: Translation and validation of the standard Chinese version of the EORTC QLQ-C30. Qual. Life Res. 9, 129–137 (2000). https://doi.org/10.1023/A:1008981520920
Finch, A.P., Gamper, E., Norman, R., et al.: Estimation of an EORTC QLU-C10 value set for Spain using a discrete choice experiment. Pharmacoeconomics 39, 1085–1098 (2021). https://doi.org/10.1007/s40273-021-01058-x
HKSAR Government. Hong Kong Population By-census (2016). http://www.bycensus2016.gov.hk/en/bc-mt.html. Accessed 13 Oct 2021
Lancsar, E., Louviere, J.: Conducting discrete choice experiments to inform healthcare decision making: a users guide. Pharmacoeconomics 26, 661–677 (2008). https://doi.org/10.2165/00019053-200826080-00004
Norman, R., Viney, R., Aaronson, N.K., et al.: Using a discrete choice experiment to value the QLU-C10D: feasibility and sensitivity to presentation format. Qual. Life Res. 25, 637–649 (2016). https://doi.org/10.1007/s11136-015-1115-3
Jansen, F., Verdonck-de Leeuw, I.M., Gamper, E., et al.: Dutch utility weights for the EORTC cancer-specific utility instrument: the Dutch EORTC QLU-C10D. Qual. Life Res. (2021). https://doi.org/10.1007/s11136-021-02767-8
Deming, W.E., Stephan, F.F.: On a least squares adjustment of a sampled frequency table when the expected marginal totals are known. Ann. Math. Stat. 11, 427–444 (1940). https://doi.org/10.1214/aoms/1177731829
Gu, Y., Norman, R., Viney, R.: Estimating health state utility values from discrete choice experiments—a QALY space model approach. Health Econ. 23, 1098–1114 (2014). https://doi.org/10.1002/hec.3066
Hong Kong Food and Health Bureau. Mental health review report (2018). https://www.healthbureau.gov.hk/download/press_and_publications/otherinfo/180500_mhr/e_mhr_full_report.pdf. Accessed 25 Dec 2021
Kilkkinen, A., Kao-Philpot, A., O’Neil, A., et al.: Prevalence of psychological distress, anxiety and depression in rural communities in Australia. Aust. J. Rural Health 15, 114–119 (2007). https://doi.org/10.1111/j.1440-1584.2007.00863.x
Mental Health Foundation. Mental health statistics: depression. Mental Health Foundation. https://www.mentalhealth.org.uk/statistics/mental-health-statistics-depression. Accessed 30 Mar 2022
Mental health in Spain. https://www.statista.com/topics/8060/mental-health-in-spain/#dossierKeyfigures. Accessed 29 Dec 2021
Stratton, E., Lampit, A., Choi, I., et al.: Effectiveness of eHealth interventions for reducing mental health conditions in employees: a systematic review and meta-analysis. PLoS ONE 12, e0189904 (2017)
Wong, D.K.-K., Cheung, M.-K.: Online health information seeking and eHealth literacy among patients attending a primary care clinic in Hong Kong: a cross-sectional survey. J. Med. Internet Res. 21, e10831 (2019). https://doi.org/10.2196/10831
Rowen, D., Azzabi Zouraq, I., Chevrou-Severac, H., et al.: International regulations and recommendations for utility data for health technology assessment. Pharmacoeconomics 35, 11–19 (2017). https://doi.org/10.1007/s40273-017-0544-y
Kennedy-Martin, M., Slaap, B., Herdman, M., et al.: Which multi-attribute utility instruments are recommended for use in cost-utility analysis? A review of national health technology assessment (HTA) guidelines. Eur. J. Health Econ. 21, 1245–1257 (2020). https://doi.org/10.1007/s10198-020-01195-8
Chiang, C.-L., Chan, S.-K., Lee, S.-F., et al.: First-line atezolizumab plus bevacizumab versus sorafenib in hepatocellular carcinoma: a cost-effectiveness analysis. Cancers (Basel) (2021). https://doi.org/10.3390/cancers13050931
Lee, S.F., Choi, H.C.W., Chan, S.K., et al.: Cost-effectiveness of anti-epidermal growth factor receptor therapy versus bevacizumab in KRAS wild-type (WT), Pan-RAS WT, and Pan-RAS WT left-sided metastatic colorectal cancer. Front. Oncol. (2021). https://doi.org/10.3389/fonc.2021.651299
You, J.H.S., Cho, W.C.S., Ming, W., et al.: EGFR mutation-guided use of afatinib, erlotinib and gefitinib for advanced non-small-cell lung cancer in Hong Kong—a cost-effectiveness analysis. PLoS ONE 16, 1–14 (2021). https://doi.org/10.1371/journal.pone.0247860
Lai, X.B., Ching, S.S.Y., Wong, F.K.Y., et al.: The cost-effectiveness of a nurse-led care program for breast cancer patients undergoing outpatient-based chemotherapy—a feasibility trial. Eur. J. Oncol. Nurs. 36, 16–25 (2018). https://doi.org/10.1016/j.ejon.2018.07.001
Wong, E.L.Y., Ramos-Goñi, J.M., Cheung, A.W.L., et al.: Assessing the use of a feedback module to model EQ-5D-5L health states values in Hong Kong. Patient 11, 235–247 (2018). https://doi.org/10.1007/s40271-017-0278-0
Xu, R.H., Keetharuth, A.D., Wang, L., et al.: Psychometric evaluation of the Chinese Recovering Quality of Life (ReQoL) outcome measure and assessment of health-related quality of life during the COVID-19 pandemic. Front. Psychol. (2021). https://doi.org/10.3389/fpsyg.2021.663035
Funding
This study was supported by the European Organisation for Research and Treatment of Cancer Quality of Life Group, Grant No. 001/2018.
Author information
Authors and Affiliations
Consortia
Contributions
Concept and design: EW, RN, JL, BH, GK, and MK. Acquisition of data: GK. Analysis and interpretation of data: RX, RN, JL, BN, and GK. Drafting of the manuscript: RX and LN. Critical revision of paper for important intellectual content: RX, EW, LN, RN, BH, JL, GK, and MK. Statistical analysis: GK. Provision of study materials or patients: MK. Obtaining funding: BH and GK. Administrative, technical, or logistic support: JL. Supervision: EW.
Corresponding author
Ethics declarations
Conflict of interest
RX, EW, NL, RN, BH, and HT has nothing to disclose. JL and GK reports grants from EORTC Quality of Life Group during the conduct of the study. MK reports grants from the Australian National Health and Medical Research Council (NHMRC Project Grant 632662, to develop the QLU-C10D descriptive system and valuation methods used in the current study) and from the EORTC QOL Group for the conduct of the current study.
Ethics approval
The study was approved by the Ethics Committee, Hong Kong Polytechnic University, with approval number HSEARS20220523001.
Informed consent
Informed consent was collected from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, R.H., Wong, E.Ly., Luo, N. et al. The EORTC QLU-C10D: the Hong Kong valuation study. Eur J Health Econ (2023). https://doi.org/10.1007/s10198-023-01632-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10198-023-01632-4
Keywords
- Utility weights
- Discrete choice experiment
- EORTC QLQ-C30
- QLU-C10D
- Hong Kong
- Quality of life
- Decision making