Abstract
Objectives
Challenges with patient-reported outcome (PRO) evidence and health state utility values (HSUVs) in rare diseases exist due to small, heterogeneous populations, lack of disease knowledge and early onset. To better incorporate quality of life (QoL) into Health Technology Assessment, a clearer understanding of these challenges is needed.
Methods
NICE appraisals of non-oncology treatments with an EMA orphan designation (n = 24), and corresponding appraisals in the Netherlands, France, and Germany were included. Document analysis of appraisal reports investigated how PROs/HSUVs influenced decision-making and was representative of QoL impact of condition and treatment.
Results
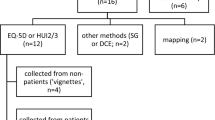
PRO evidence was not included in 6/24 NICE appraisals. When included, it either failed to demonstrate change, capture domains important for patients, or was uncertain. In the other countries, little information was reported and evidence largely did not demonstrate change. In NICE appraisals, HSUVs were derived through the collection of EQ-5D data (7/24 cases), mapping (6/24), vignettes (5/24), and published literature or other techniques (6/24). The majority did not use data collected alongside clinical trials. Few measures demonstrated significant change due to lack of sensitivity or face validity, short-term data, or implausible health states. In 8/24 NICE appraisals, patient surveys or input during appraisal committee meetings supported the interpretation of uncertainty or provided evidence about QoL.
Conclusions
This study sheds light on the nature of PRO evidence in rare diseases and associated challenges. Results emphasise the need for improved development and use of PRO/HSUVs. Other forms of evidence and expert input are crucial to support better appraisal of uncertain or missing evidence.

Similar content being viewed by others
Availability of data and materials
Data were extracted from publicly available reports and literature.
References
European Commission: Orphan medicinal products, https://ec.europa.eu/health/human-use/orphan-medicines_en
Eurordis: About rare diseases, https://www.eurordis.org/about-rare-diseases
Bogart, K.R., Irvin, V.L.: Health-related quality of life among adults with diverse rare disorders. Orphanet J. Rare Dis. (2017). https://doi.org/10.1186/s13023-017-0730-1
Drummond, M., Sculpher, M., Claxton, K., Stoddart, G., Torrance, G.: Measuring and valuing health effects. In: Methods for economic evaluation in health care. pp. 123–180. Oxford University Press, Oxford (2015)
FDA: Guidance for industry - Patient-reported outcome measures: use in medical product development to support labeling claims. (2009)
Kingsley, C., Patel, S.: Patient-reported outcome measures and patient-reported experience measures. BJA Educ. 17, 137–144 (2017). https://doi.org/10.1093/bjaed/mkw060
Brazier, J., Ara, R., Azzabi, I., Busschbach, J., Chevrou-Séverac, H., Crawford, B., Cruz, L., Karnon, J., Lloyd, A., Paisley, S., Pickard, A.S.: Identification, review, and use of health state utilities in cost-effectiveness models: an ISPOR good practices for outcomes research task force report. Value Heal. (2019). https://doi.org/10.1016/j.jval.2019.01.004
Whittal, A., Meragaglia, M., Nicod, E.: The use of patient-reported outcome measures (PROMs) in rare diseases and implications for HTA. Patient. under review (2020)
Benjamin, K., Vernon, M.K., Patrick, D.L., Perfetto, E., Nestler-Parr, S., Burke, L.: Patient-reported outcome and observer-reported outcome assessment in rare disease clinical trials: an ISPOR COA emerging good practices task force report. Value Heal. (2017). https://doi.org/10.1016/j.jval.2017.05.015
Slade, A., Isa, F., Kyte, D., Pankhurst, T., Kerecuk, L., Ferguson, J., Lipkin, G., Calvert, M.: Patient reported outcome measures in rare diseases: a narrative review, (2018)
Pearson, I., Rothwell, B., Olaye, A., Knight, C.: Economic modeling considerations for rare diseases. Value Heal. 21, 515–524 (2018). https://doi.org/10.1016/j.jval.2018.02.008
Towse, A., Garau, M.: Appraising ultra-orphan drugs: is cost-per-QALY appropriate? A review of the evidence. (2018)
Annemans, L., Aymé, S., Le Cam, Y., Facey, K., Gunther, P., Nicod, E., Reni, M., Roux, J.-L., Schlander, M., Taylor, D., Tomino, C., Torrent-Farnell, J., Upadhyaya, S., Hutchings, A., Le Dez, L.: Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL). Orphanet J. Rare Dis. 12, 50 (2017). https://doi.org/10.1186/s13023-017-0601-9
Gutierrez, L., Patris, J., Hutchings, A., Cowell, W.: Principles for consistent value assessment and sustainable funding of orphan drugs in Europe. Orphanet J. Rare Dis. 10, 53 (2015). https://doi.org/10.1186/s13023-015-0269-y
Kleijnen, S., Leonardo Alves, T., Meijboom, K., Lipska, I., De Boer, A., Leufkens, H.G., Goettsch, W.G.: The impact of quality-of-life data in relative effectiveness assessments of new anti-cancer drugs in European countries. Qual. Life Res. (2017). https://doi.org/10.1007/s11136-017-1574-9
National Institute for Health and Care Excellence.: Asfotase alfa for treating paediatric-onset hypophosphatasia. HST6. (2017)
National Institute for Health and Care Excellence.: Eculizumab for treating atypical haemolytic uraemic syndrome. HST1. 2015.
National Institute for Health and Care Excellence.: Colistimethate sodium and tobramycin dry powders for inhalation for treating pseudomonas lung infection in cystic fibrosis. TA276. (2013)
National Institute for Health and Care Excellence.: Migalastat for treating Fabry disease. HST4. (2017)
National Institute for Health and Care Excellence.: Inotersen for treating hereditary transthyretin amyloidosis. HST9. (2019)
National Institute for Health and Care Excellence.: Voretigene neparvovec for treating inherited retinal dystrophies caused by RPE65 gene mutations. HST11. (2019)
National Institute for Health and Care Excellence.: Mannitol dry powder for inhalation for treating cystic fibrosis. TA266. (2012)
National Institute for Health and Care Excellence.: Lumacaftor–ivacaftor for treating cystic fibrosis homozygous for the F508del mutation. TA398. (2016)
National Institute for Health and Care Excellence.: Elosulfase alfa for treating mucopolysaccharidosis type IVa. HST2. (2015)
National Institute for Health and Care Excellence.: Burosumab for treating X-linked hypophosphataemia in children and young people. HST8. (2018)
National Institute for Health and Care Excellence.: Strimvelis for treating adenosine deaminase deficiency–severe combined immunodeficiency. HST7. (2018)
National Institute for Health and Care Excellence.: Nusinersen for treating spinal muscular atrophy. TA588. (2019)
National Institute for Health and Care Excellence.: Letermovir for preventing cytomegalovirus disease after a stem cell transplant. TA591. (2019)
National Institute for Health and Care Excellence.: Mepolizumab for treating severe refractory eosinophilic asthma. TA431. (2017)
National Institute for Health and Care Excellence.: Patisiran for treating hereditary transthyretin amyloidosis. HST10. (2019)
National Institute for Health and Care Excellence.: Darvadstrocel for treating complex perianal fistulas in Crohn’s disease. TA556. (2019)
National Institute for Health and Care Excellence.: Eliglustat for treating type 1 Gaucher disease. HST5. (2017)
National Institute for Health and Care Excellence.: Holoclar for treating limbal stem cell deficiency after eye burns. TA467. (2017)
National Institute for Health and Care Excellence.: Lanadelumab for preventing recurrent attacks of hereditary angioedema. TA606. (2019)
National Institute for Health and Care Excellence.: Obeticholic acid for treating primary biliary cholangitis. TA443. (2017)
National Institute for Health and Care Excellence.: Cerliponase alfa for treating neuronal ceroid lipofuscinosis type 2. HST12. (2019)
National Institute for Health and Care Excellence.: Pirfenidone for treating idiopathic pulmonary fibrosis. TA504. (2018)
National Institute for Health and Care Excellence.: Nintedanib for treating idiopathic pulmonary fibrosis. TA379. (2016)
National Institute for Health and Care Excellence.: Ataluren for treating Duchenne muscular dystrophy with a nonsense mutation in the dystrophin gene. HST3. (2016)
Nicod, E., Kanavos, P.: Developing an evidence-based methodological framework to systematically compare HTA coverage decisions: A mixed methods study, (2016)
Bryman, A.: Social Research Methods, Oxford (2004)
Lerman, J.A., Sullivan, E., Barnes, D.A., Haynes, R.J.: The Pediatric Outcomes Data Collection Instrument (PODCI) and functional assessment of patients with unilateral upper extremity deficiencies. J. Pediatr. Orthop. (2005). https://doi.org/10.1097/01.bpo.0000149866.80894.70
Jones, P.W., Quirk, F.H., Baveystock, C.M.: The St George’s Respiratory Questionnaire. Respir. Med. (1991). https://doi.org/10.1016/S0954-6111(06)80166-6
Meregaglia, M., Nicod, E., Drummond, M.: The estimation of health state utility values in rare diseases: overview of existing techniques. Int. J. Technol. Assess. Health Care. (2020). https://doi.org/10.1017/S0266462320000665
Guidance to submitting companies for completion of new product assessment form (NPAF). Supplement for medicines for extremely rare conditions (ultra-orphan medicines). , Glasgow (2019)
Beusterien, K., Leigh, N., Jackson, C., Miller, R., Mayo, K., Revicki, D.: Integrating preferences into health status assessment for amyotrophic lateral sclerosis: the ALS Utility Index. Amyotroph. Lateral Scler. 6, 169–176 (2005). https://doi.org/10.1080/14660820410021339
Rüther, A., Elstein, D., Wong-Rieger, D., Guyatt, G.: Aspects of patient reported outcomes in rare diseases: a discussion paper. Int. J. Technol. Assess. Health Care. 32, 126–130 (2016). https://doi.org/10.1017/S0266462316000271
Schulz, S., Passon, A., Kulig, M., Perleth, M., Matthias, K.: Orphan drug benefits asssessments at the Federal Joint Committee in Germany. In: HTAi conference (2019)
Bell, J.A., Galaznik, A., Pompilus, F., Strzok, S., Bejar, R., Scipione, F., Fram, R.J., Faller, D.V., Cano, S., Marquis, P.: A pragmatic patient-reported outcome strategy for rare disease clinical trials: application of the EORTC item library to myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J. Patient-Rep. Outcomes. (2019). https://doi.org/10.1186/s41687-019-0123-4
Morel, T., Cano, S.J.: Measuring what matters to rare disease patients—reflections on the work by the IRDiRC taskforce on patient-centered outcome measures. Orphanet J. Rare Dis. (2017). https://doi.org/10.1186/s13023-017-0718-x
Goodrich, K., Kaambwa, B., Al-Janabi, H.: The inclusion of informal care in applied economic evaluation: a review. Value Heal. (2012). https://doi.org/10.1016/j.jval.2012.05.009
Pennington, B., Wong, R.: Modelling carer health-related quality of life in NICE Technology Appraisals and Highly Specialised Technologies. (2019)
Acknowledgements
We would like to thank Dr. Andrew Lloyd for his time and feedback on the results.
Funding
This research was funded by the European Commission’s Horizon 2020 research and innovation programme and was undertaken under the auspices of IMPACT-HTA (Grant # 779312). The results presented here reflect the authors’ views and not the views of the European Commission. The European Commission is not liable for any use of the information communicated. No funds, grants or other support was received.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the design of the work; EN and AW collected the data, EN conducted the data analysis and drafted the work; all authors revised it critically at several occasions for important intellectual content; all authors approved the version being submitted; all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
Michael Drummond and Karen Facey have received funding and consultancy fees from manufacturers of treatments for rare diseases outside of this work. Elena Nicod and Amanda Whittal are part-time employed by Dolon Ltd and have no conflicts with this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nicod, E., Meregaglia, M., Whittal, A. et al. Consideration of quality of life in the health technology assessments of rare disease treatments. Eur J Health Econ 23, 645–669 (2022). https://doi.org/10.1007/s10198-021-01387-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-021-01387-w
Keywords
- Patient-reported outcome
- Rare disease
- Orphan medicinal products
- Health-state utility value
- Health technology assessment
- Reimbursement