Abstract
Background
Previous studies on adjuvant chemotherapy for patients with ovarian clear cell carcinoma (OCCC) have included a limited number of Asian patients with surgical stage I OCCC, despite differences in OCCC survival by race and stage. The aim of this study was to estimate the survival effect of the number of cycles of adjuvant taxane plus carboplatin chemotherapy in Asian patients with surgical stage I OCCC.
Methods
We retrospectively identified 227 patients with surgical stage I OCCC at 14 institutions from 1995 to 2017. Kaplan–Meier analysis and Cox proportional hazard regression with inverse probability of treatment weighting (IPTW) adjustment were performed to evaluate overall survival (OS) and recurrence-free survival (RFS) in patients receiving ≤ 3 and 4–6 cycles of taxane plus platinum adjuvant chemotherapy.
Results
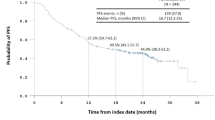
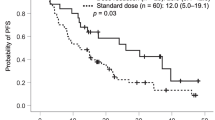
Eighty-nine and 138 patients received ≤ 3 and 4–6 cycles of adjuvant chemotherapy, respectively. There was no between-group difference in OS or RFS with or without IPTW adjustment. In Cox proportional hazards analysis, 4–6 cycles of adjuvant chemotherapy were not associated with improved OS (HR 1.090; 95% CI 0.518–2.291; p = 0.821) or RFS (HR 1.144; 95% CI 0.619–2.114; p = 0.669) compared to ≤ 3 cycles, even with IPTW adjustment. Subgroup analysis in different substages of stage I OCCC showed that the number of cycles of adjuvant chemotherapy had no impact on OS or RFS.
Conclusion
Three or fewer cycles of taxane plus carboplatin chemotherapy may be a reasonable treatment regime for patients with surgical staging I OCCC.



Similar content being viewed by others
References
Torre LA, Trabert B, DeSantis CE et al (2018) Ovarian cancer statistics, 2018. CA Cancer J Clin 68(4):284–296. https://doi.org/10.3322/caac.21456
Peres LC, Sinha S, Townsend MK et al (2020) Predictors of survival trajectories among women with epithelial ovarian cancer. Gynecol Oncol 156(2):459–466. https://doi.org/10.1016/j.ygyno.2019.12.011
Fujiwara K, Shintani D, Nishikawa T (2016) Clear-cell carcinoma of the ovary. Ann Oncol Off J Eur Soc Med Oncol 27(Suppl 1):i50–i52. https://doi.org/10.1093/annonc/mdw086
Nagase S, Ohta T, Takahashi F et al (2019) Annual report of the committee on gynecologic oncology, the Japan Society of Obstetrics and Gynecology: annual patients report for 2015 and annual treatment report for 2010. J Obstet Gynaecol Res 45(2):289–298. https://doi.org/10.1111/jog.13863
Sugiyama T (2000) Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 88:2584–2589
Mabuchi S, Sugiyama T, Kimura T (2016) Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives. J Gynecol Oncol 27(3):e31. https://doi.org/10.3802/jgo.2016.27.e31
Lorusso D, Pignata S (2017) Role of adjuvant chemotherapy in early-stage endometrioid and clear-cell ovarian cancer. Ann Oncol Off J Eur Soc Med Oncol 28(12):2909–2911. https://doi.org/10.1093/annonc/mdx539
Magazzino F, Katsaros D, Ottaiano A et al (2011) Surgical and medical treatment of clear cell ovarian cancer: results from the multicenter Italian Trials in Ovarian Cancer (MITO) 9 retrospective study. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc 21(6):1063–1070. https://doi.org/10.1097/IGC.0b013e318218f270
Network NCC (2017) National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. version 4.2017. https://www2.tri-kobe.org/nccn/guideline/gynecological/english/ovarian.pdf. Accessed 14 Nov 2021
Trimbos JB, Parmar M, Vergote I et al (2003) International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy in Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst 95(2):105–112
Trimbos JB, Vergote I, Bolis G et al (2003) Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J Natl Cancer Inst 95(2):113–125
Hogen L, Brar H, Covens A et al (2017) Is adjuvant chemotherapy beneficial for surgical stage I ovarian clear cell carcinoma? Gynecol Oncol 147(1):54–60. https://doi.org/10.1016/j.ygyno.2017.07.128
Mizuno M, Kajiyama H, Shibata K et al (2012) Adjuvant chemotherapy for stage I ovarian clear cell carcinoma: is it necessary for stage IA? Int J Gynecol Cancer Off J Int Gynecol Cancer Soc 22(7):1143–1149. https://doi.org/10.1097/IGC.0b013e31825c7cbe
Oseledchyk A, Leitao MM Jr, Konner J et al (2017) Adjuvant chemotherapy in patients with stage I endometrioid or clear cell ovarian cancer in the platinum era: a Surveillance, Epidemiology, and End Results Cohort Study, 2000–2013. Ann Oncol Off J Eur Soc Med Oncol 28(12):2985–2993. https://doi.org/10.1093/annonc/mdx525
Takada T, Iwase H, Iitsuka C et al (2012) Adjuvant chemotherapy for stage I clear cell carcinoma of the ovary: an analysis of fully staged patients. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc 22(4):573–578. https://doi.org/10.1097/IGC.0b013e31823fd413
Takano M, Sugiyama T, Yaegashi N et al (2010) Less impact of adjuvant chemotherapy for stage I clear cell carcinoma of the ovary: a retrospective Japan Clear Cell Carcinoma Study. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc 20(9):1506–1510. https://doi.org/10.1111/IGC.0b013e3181fcd089
Nasioudis D, Mastroyannis SA, Albright BB et al (2018) Adjuvant chemotherapy for stage I ovarian clear cell carcinoma: patterns of use and outcomes. Gynecol Oncol 150(1):14–18. https://doi.org/10.1016/j.ygyno.2018.04.567
Liu SL, Tinker AV (2020) Omission of adjuvant therapy in stage I clear cell ovarian cancer: review of the BC cancer experience. Gynecol Oncol Rep 31:100533. https://doi.org/10.1016/j.gore.2019.100533
Bogani G, Ditto A, Lopez S et al (2020) Adjuvant chemotherapy vs. observation in stage I clear cell ovarian carcinoma: a systematic review and meta-analysis. Gynecol Oncol 157(1):293–298. https://doi.org/10.1016/j.ygyno.2019.12.045
Chan JK, Tian C, Fleming GF et al (2010) The potential benefit of 6 vs. 3 cycles of chemotherapy in subsets of women with early-stage high-risk epithelial ovarian cancer: an exploratory analysis of a Gynecologic Oncology Group study. Gynecol Oncol 116(3):301–306. https://doi.org/10.1016/j.ygyno.2009.10.073
Prendergast EN, Holzapfel M, Mueller JJ et al (2017) Three versus six cycles of adjuvant platinum-based chemotherapy in early stage clear cell ovarian carcinoma—a multi-institutional cohort. Gynecol Oncol 144(2):274–278. https://doi.org/10.1016/j.ygyno.2016.12.004
Prat J (2014) Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet 124(1):1–5. https://doi.org/10.1016/j.ijgo.2013.10.001
Chen VW, Ruiz B, Killeen JL et al (2003) Pathology and classification of ovarian tumors. Cancer 97(10 Suppl):2631–2642. https://doi.org/10.1002/cncr.11345
Rustin GJ, Vergote I, Eisenhauer E et al (2011) Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer Off J Int Gynecol Cancer Soc 21(2):419–423. https://doi.org/10.1097/IGC.0b013e3182070f17
Higashi M, Kajiyama H, Shibata K et al (2011) Survival impact of capsule rupture in stage I clear cell carcinoma of the ovary in comparison with other histological types. Gynecol Oncol 123(3):474–478. https://doi.org/10.1016/j.ygyno.2011.08.036
Kajiyama H, Suzuki S, Yoshihara M et al (2018) The possible existence of occult metastasis in patients with ovarian clear-cell carcinoma who underwent complete resection without any residual tumours. Oncotarget 9(5):6298–6307. https://doi.org/10.18632/oncotarget.23921
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Matsuo K, Yoshino K, Hasegawa K et al (2015) Survival outcome of stage I ovarian clear cell carcinoma with lympho-vascular space invasion. Gynecol Oncol 136(2):198–204. https://doi.org/10.1016/j.ygyno.2014.12.006
Bell J, Brady MF, Young RC et al (2006) Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 102(3):432–439. https://doi.org/10.1016/j.ygyno.2006.06.013
Pectasides D, Fountzilas G, Aravantinos G et al (2006) Advanced stage clear-cell epithelial ovarian cancer: the Hellenic Cooperative Oncology Group experience. Gynecol Oncol 102(2):285–291. https://doi.org/10.1016/j.ygyno.2005.12.038
Parte SC, Batra SK, Kakar SS (2018) Characterization of stem cell and cancer stem cell populations in ovary and ovarian tumors. J Ovarian Res 11(1):69. https://doi.org/10.1186/s13048-018-0439-3
Oza AM, Cook AD, Pfisterer J et al (2015) Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol 16(8):928–936. https://doi.org/10.1016/s1470-2045(15)00086-8
Acknowledgements
The authors sincerely thank TOTSG members for collaborating in the data collection.
Funding
This research received no specific grant from any funding agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The study was approved by the ethics review board of Nagoya University (approval number 357-3).
Informed consent
For this study, the IRB issued a waiver for written consent because data collection was retrospective.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10147_2021_2075_MOESM1_ESM.pptx
Supplementary Fig. 1 Survival difference in Kaplan–Meier-estimated OS (a) and RFS (b) curves in stage IA OCCC patients who received 0, 1–3, or 4–6 cycles of adjuvant chemotherapy. p-values were estimated using the log-rank test. OCCC, ovarian clear cell carcinoma; OS, overall survival; RFS, recurrence-free survival; CI, confidence interval. Supplementary Fig. 2 Survival differences in stage IC OCCC. Kaplan–Meier-estimated OS and RFS curves in stage IC (a, b) OCCC patients who received 0, 1–3, or 4–6 cycles of adjuvant chemotherapy and stage IC2/IC3 (c, d) OCCC patients who received, ≤ 3 or 4–6 cycles of adjuvant chemotherapy. p-values were estimated using the log-rank test. OCCC, ovarian clear cell carcinoma; OS, overall survival; RFS, recurrence-free survival; CI, confidence interval. (PPTX 160 kb)
About this article
Cite this article
Ukai, M., Suzuki, S., Yoshihara, M. et al. Adjuvant taxane plus platinum chemotherapy for stage I ovarian clear cell carcinoma with complete surgical staging: are more than three cycles necessary?. Int J Clin Oncol 27, 609–618 (2022). https://doi.org/10.1007/s10147-021-02075-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02075-8