Abstract
Background
This study aimed to investigate the clinical benefit of dose-dense paclitaxel plus carboplatin (TC) with bevacizumab therapy for advanced ovarian, fallopian tube, and primary peritoneal cancer patients in the neoadjuvant setting.
Methods
Ovarian, fallopian tube or primary peritoneal cancer patients with stage III–IV disease received neoadjuvant chemotherapy (NAC) every 3 weeks consisting of paclitaxel (80 mg/m2) on days 1, 8, and 15; carboplatin (AUC 6.0 mg/mL × min.) on day 1; and bevacizumab (15 mg/kg) on day 1. Interval debulking surgery (IDS) was performed after 3 cycles of dose-dense TC-bevacizumab therapy. The primary endpoint was the rate of complete resection by IDS. Secondary endpoints were treatment completion rate, treatment exposure, response rate to NAC, adverse events, and perioperative complications.
Results
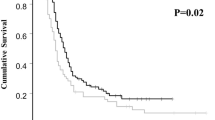
Twenty-four patients were included in this study. The median age was 55.5 years (37–80 years), and most patients had high-grade serous carcinoma accounted (n = 18). IDS was performed in all patients with complete resection achieved in 75% (95% confidence interval: 57.7–92.3%). The lower limit exceeded the preset threshold rate of 55%. The response rate to NAC was 79%, and serum CA125 levels were in the normal range after NAC in 57% of patients. Grade 4 hematological toxicities and grade 3/4 non-hematological toxicities occurred in 29% and 17% of patients during NAC, respectively. Grade 3/4 perioperative complications were seen in 29% of patients, but no gastrointestinal perforations or treatment-related deaths occurred.
Conclusions
Neoadjuvant dose-dense TC-bevacizumab therapy was well tolerated, and a satisfactory rate of complete resection by IDS was achieved.
Similar content being viewed by others
References
du Bois A, Reuss A, Pujade-Lauraine E et al (2009) Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 115:1234–1244
Bristow RE, Chi DS (2006) Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol 103:1070–1076
Kang S, Nam BH (2009) Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann Surg Oncol 16:2315–2320
Vergote I, Tropé CG, Amant F et al (2010) European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363:943–953
Kehoe S, Hook J, Nankivell M et al (2015) Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 386:249–257
Vergote I, Coens C, Nankivell M et al (2018) EORTC; MRC CHORUS study investigators. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol 19:1680–1687
Katsumata N, Yasuda M, Takahashi F et al (2009) Japanese Gynecologic Oncology Group. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374:1331–1338
Burger RA, Brady MF, Bookman MA et al (2011) Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473–2483
Perren TJ, Swart AM, Pfisterer J et al (2011) ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484–2496
Gonzalez-Martin A, Gladieff L, Tholander B et al (2013) OCTAVIA Investigators. Efficacy and safety results from OCTAVIA, a single-arm phase II study evaluating front-line bevacizumab, carboplatin and weekly paclitaxel for ovarian cancer. Eur J Cancer 49:3831–3838
Onda T, Satoh T, Saito T et al (2016) Japan Clinical Oncology Group. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer 264:22–31
Onda T, Satoh T, Ogawa G et al (2020) Japan Clinical Oncology Group. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur J Cancer 130:114–125
Fagotti A, Ferrandina G, Vizzielli G et al (2016) Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of peri-operative outcome. Eur J Cancer 59:22–33
Yoshihama T, Nomura H, Iwasa N et al (2017) Efficacy and safety of dose-dense paclitaxel plus carboplatin as neoadjuvant chemotherapy for advanced ovarian, fallopian tube or peritoneal cancer. Jpn J Clin Oncol 47:1019–1023
Ebata T, Yunokawa M, Bun S et al (2016) Dose-dense paclitaxel plus carboplatin as neoadjuvant chemotherapy for advanced ovarian, fallopian tube, or primary peritoneal carcinomas. Cancer Chemother Pharmacol 78:1283–1288
Rouzier R, Gouy S, Selle F et al (2017) Efficacy and safety of bevacizumab-containing neoadjuvant therapy followed by interval debulking surgery in advanced ovarian cancer: results from the ANTHALYA trial. Eur J Cancer 70:133–142
Petrillo M, Paris I, Vizzielli G et al (2015) Neoadjuvant chemotherapy followed by maintenance therapy with or without bevacizumab in unresectable high-grade serous ovarian cancer: a case-control study. Ann Surg Oncol 22:952–958
Komiyama S, Kugimiya T, Kubushiro K (2018) Safety and efficacy of neoadjuvant chemotherapy containing bevacizumab and interval debulking surgery for advanced epithelial ovarian cancer: a feasibility study. J Surg Oncol 118:687–693
Ferriss JS, Java JJ, Bookman MA et al (2015) Ascites predicts treatment benefit of bevacizumab in front-line therapy of advanced epithelial ovarian, fallopian tube and peritoneal cancers: an NRG Oncology/GOG study. Gynecol Oncol 139:17–22
Daniele G, Lorusso D, Scambia G et al (2017) Feasibility and outcome of interval debulking surgery (IDS) after carboplatin-paclitaxel-bevacizumab (CPB): a subgroup analysis of the MITO-16A-MaNGO OV2A phase 4 trial. Gynecol Oncol 144:256–259
Komiyama S, Kato K, Inokuchi Y et al (2019) Bevacizumab combined with platinum-taxane chemotherapy as first-line treatment for advanced ovarian cancer: a prospective observational study of safety and efficacy in Japanese patients (JGOG3022 trial). Int J Clin Oncol 24:103–114
Moore K, Colombo N, Scambia G et al (2018) Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495–2505
González-Martín A, Pothuri B, Vergote I et al (2019) PRIMA/ENGOT-OV26/GOG-3012 investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391–2402
Onda T, Kobayashi H, Nakanishi T et al (2009) Feasibility study of neoadjuvant chemotherapy followed by interval debulking surgery for stage III/IV ovarian, tubal, and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0206. Gynecol Oncol 113:57–62
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP18K09297.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hiroyuki Nomura reports grants from Japan Society for the Promotion of Science, during the conduct of the study. All other authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Iwasa-Inoue, N., Nomura, H., Kataoka, F. et al. Prospective feasibility study of neoadjuvant dose-dense paclitaxel plus carboplatin with bevacizumab therapy followed by interval debulking surgery for advanced ovarian, fallopian tube, and primary peritoneal cancer patients. Int J Clin Oncol 27, 441–447 (2022). https://doi.org/10.1007/s10147-021-02050-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02050-3