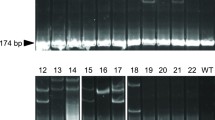
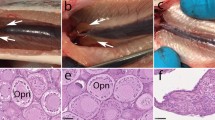
Abstract
The use of sterile recipients is crucial for efficiently producing donor-derived offspring through surrogate broodstock technology for practical aquaculture applications. Although knockout (KO) of the dead end (dnd) gene has been used in previous studies as a sterilization method, it has not been reported in marine fish. In this study, nibe croaker was utilized as a model for marine teleosts that produce small pelagic eggs, and the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (CRISPR/Cas9) system was utilized to produce dnd KO fish. The F1 generation, which carried a nonsense mutation in the dnd gene, was produced by mating founder individuals with wild-type counterparts. Subsequently, the F2 generation was produced by mating the resulting males and females. Among the F2 generations, 24.0% consisted of homozygous KO individuals. Histological analysis revealed that primordial germ cells (PGCs) were present in homozygous KO individuals at 10 days post-hatching (dph), similar to wild-type individuals. However, by 20 dph, PGCs were absent in KO individuals. Furthermore, no germ cells were observed in the gonads of both sexes of homozygous KO individuals at 6 months old, which is the typical maturity age for wild-type individuals of both sexes. In addition, when cryopreserved donor nibe croaker testicular cells were transplanted, only donor-derived offspring were successfully obtained through the spontaneous mating of homozygous KO recipient parents. Results indicate that dnd KO nibe croaker lacks germ cells and can serve as promising recipients, producing only donor-derived gametes as surrogate broodstock.









Similar content being viewed by others
Data Availability
The data supporting the findings of this study are available upon request.
References
Baloch R, Franěk R, Saito T, Pšenička M (2021) Dead-end (dnd) protein in fish—a review. Fish Physiol Biochem 47(3):777–784. https://doi.org/10.1007/s10695-018-0606-x
Cárdenas S (2012) Biología y acuicultura de corvinas en el mundo. AquaTIC 37:1–13
Drum M, Kranaster R, Ewald C, Blasczyk R, Marx A (2014) Variants of a Thermus aquaticus DNA polymerase with increased selectivity for applications in allele- and methylation-specific amplification. PLoS ONE 9:e96640
FAO (1950–2020) (FishstatJ) [Internet] Fishery and aquaculture statistics. Global aquaculture production. Rev Aquacult Page 42 of 74 For Review Only [actualizado 2023; citado el 2 de abril de 2023]. Disponible en: http://www.fao.org/fishery/statistics/software/fishstatj/en. Roma, Italia: FAO Fisheries and Aquaculture Department 2023
Franěk R, Kašpar V, Shah MA, Gela D, Pšenička M (2021) Production of common carp donor-derived offspring from goldfish surrogate broodstock. Aquaculture 534:736252
Fujimoto T, Nishimura T, Goto-Kazeto R, Kawakami Y, Yamaha E, Arai K (2010) Sexual dimorphism of gonadal structure and gene expression in germ cell-deficient loach, a teleost fish. Proc Natl Acad Sci USA 107:17211–17216
Fujihara R, Katayama N, Sadaie S, Miwa M, Sanchez Matias GA, Ichida K, Fujii W, Naito K, Hayashi M, Yoshizaki G (2022) Production of germ cell-less rainbow trout by dead end gene knockout and their use as recipients for germ cell transplantation. Mar Biotechnol (NY) 24:417–429
Goto R, Saito T, Takeda T, Fujimoto T, Takagi M, Arai K, Yamaha E (2012) Germ cells are not the primary factor for sexual fate determination in goldfish. Dev Biol 370:98–109
Gross-Thebing T, Yigit S, Pfeiffer J, Reichman-Fried M, Bandemer J, Ruckert C, Rathmer C, Goudarzi M, Stehling M, Tarbashevich K, Seggewiss J, Raz E (2017) The vertebrate protein dead end maintains primordial germ cell fate by inhibiting somatic differentiation. Dev Cell 43:704–715.e5
Güralp H, Skaftnesmo KO, Kjærner-Semb E, Straume AH, Kleppe L, Schulz RW, Edvardsen RB, Wargelius A (2020) Rescue of germ cells in dnd crispant embryos opens the possibility to produce inherited sterility in Atlantic salmon. Sci Rep 10:18042
Kawamura W, Tani R, Yahagi H, Kamio S, Morita T, Takeuchi Y, Yazawa R, Yoshizaki G (2020) Suitability of hybrid mackerel (Scomber australasicus × S. japonicus) with germ cell-less sterile gonads as a recipient for transplantation of bluefin tuna germ cells. Gen Comp Endocrinol 295:113525
Kawamura W, Hasegawa N, Yamauchi A, Kimura T, Yahagi H, Tani R, Morita T, Yazawa R, Yoshizaki G (2022) Production of albino chub mackerel (Scomber japonicus) by slc45a2 knockout and the use of a positive phototaxis-based larviculture technique to overcome the lethal albino phenotype. Aquaculture 560:738490
Kinoshita I, Fujita S (1988) Larvae and juveniles of blue drum, Nibea mitsukurii, occurring in the surf zone of Tosa Bay, Japan. Jap Jour Ich 35:25–30
Kurokawa HD, Saito D, Nakamura S, Katoh-Fukui Y, Ohta K, Baba T, Morohashi K, Tanaka M (2007) Germ cells are essential for sexual dimorphism in the medaka gonad. Proc Natl Acad Sci USA 104:16958–16963
Lee S, Iwasaki Y, Shikina S, Yoshizaki G (2013) Generation of functional eggs and sperm from cryopreserved whole testes. Proc Natl Acad Sci USA 110:1640–1645
Lee S, Katayama N, Yoshizaki G (2016) Generation of juvenile rainbow trout derived from cryopreserved whole ovaries by intraperitoneal transplantation of ovarian germ cells. Biochem Biophys Res Commun 478:1478–1483
Li Q, Fujii W, Naito K, Yoshizaki G (2017) Application of dead end-knockout zebrafish as recipients of germ cell transplantation. Mol Reprod Dev 84:1100–1111
Linhartová Z, Saito T, Kašpar V, Rodina M, Prášková E, Hagihara S, Pšenička M (2015) Sterilization of sterlet Acipenser ruthenus by using knockdown agent, antisense morpholino oligonucleotide, against dead end gene. Theriogenology 84:1246-1255.e1
Marinović Z, Li Q, Lujić J, Iwasaki Y, Csenki Z, Urbányi B, Yoshizaki G, Horváth Á (2019) Preservation of zebrafish genetic resources through testis cryopreservation and spermatogonia transplantation. Abstr Sci Rep 9(1). https://doi.org/10.1038/s41598-019-50169-1
Morishima K, Yamamoto H, Sawada Y, Miyashita S, Kato K (2009) Developing 23 new polymorphic microsatellite markers and simulating parentage assignment in the Pacific bluefin tuna, Thunnus orientalis. Mol Ecol Resour 9:790–792
Octavera A, Yoshizaki G (2019) Production of donor-derived offspring by allogeneic transplantation of spermatogonia in Chinese rosy bitterling†. Biol Reprod 100:1108–1117
Octavera A, Yoshizaki G (2020) Production of Chinese rosy bitterling offspring derived from frozen and vitrified whole testis by spermatogonial transplantation. Fish Physiol Biochem 46:1431–1442
Octavera A, Yamakawa K, Yoshizaki G (2023) The volume and shape of bitterling eggs are more strongly influenced by germ cell autonomy than by the surrounding somatic cells. Fish Physiol Biochem 49:967–981
Piferrer F (2001) Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 197:229–281
Piferrer F, Beaumont A, Falguière J, Flajšhans M, Haffray P, Colombo L (2009) Polyploid fish and shellfish: production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293:125–156
Saito T, Goto-Kazeto R, Arai K, Yamaha E (2008) Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation. Biol Reprod 78(1):159–66
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Slanchev KJ, Stebler J, de la Cueva-Méndez G, Raz E (2005) Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci USA 102:4074–4079
Takeuchi Y, Higuchi K, Yatabe T, Miwa M, Yoshizaki G (2009) Development of spermatogonial cell transplantation in Nibe croaker, Nibea mitsukurii (Perciformes, Sciaenidae). Biol Reprod 81:1055–1063
Takeuchi Y, Yatabe T, Yoshikawa H, Ino Y, Kabeya N, Yazawa R, Yoshizaki G (2018) Production of functionally sterile triploid Nibe croaker Nibea mitsukurii induced by cold-shock treatment with special emphasis on triploid aptitude as surrogate broodstock. Aquaculture 494:45–56
Takeuchi Y, Yazawa R, Yoshizaki G (2020) Chapter 17 Intraperitoneal germ cell transplantation technique in marine teleosts. In: Yoshida M, Asturiano J (eds) Reproduction in aquatic animals. Springer, Berlin
Wargelius A, Leininger S, Skaftnesmo KO, Kleppe L, Andersson E, Taranger GL, Schulz RW, Edvardsen RB (2016) Dnd knockout ablates germ cells and demonstrates germ cell independent sex differentiation in Atlantic salmon. Sci Rep 6:21284
Weidinger G, Stebler J, Slanchev K, Dumstrei K, Wise C, Lovell-Badge R, Thisse C, Thisse B, Raz E (2003) Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol 13:1429–1434
Yazawa R, Kubokawa T, Ichida K, Kawamura W, Tani R, Kamio S, Morita T, Yoshizaki G (2021) Establishment of a tracing technique for transplanted bluefin tuna germ cells in recipient’s gonads using monoclonal antibodies specifically recognizing bluefin tuna spermatogenic cells. Fish Sci 87:105–112
Yoshikawa H, Takeuchi Y, Ino Y, Wang J, Iwata G, Kabeya N, Yazawa R, Yoshizaki G (2017) Efficient production of donor-derived gametes from triploid recipients following intra-peritoneal germ cell transplantation into a marine teleost, Nibe croaker (Nibea mitsukurii). Aquaculture 478:35–47
Yoshikawa H, Xu D, Ino Y, Yoshino T, Hayashida T, Wang J, Yazawa R, Yoshizaki G, Takeuchi Y (2018) Hybrid sterility in fish caused by mitotic arrest of primordial germ cells. Genetics 209:507–521
Yoshikawa H, Ino Y, Kishimoto K, Koyakumaru H, Saito T, Kinoshita M, Yoshiura Y (2020) Induction of germ cell-deficiency in grass puffer by dead end 1 gene knockdown for use as a recipient in surrogate production of tiger puffer. Aquaculture 526:735385
Yoshizaki G, Takashiba K, Shimamori S, Fujinuma K, Shikina S, Okutsu T, Kume S, Hayashi M (2016) Production of germ cell-deficient salmonids by dead end gene knockdown, and their use as recipients for germ cell transplantation. Mol Reprod Dev 83:298–311
Yoshizaki G, Lee S (2018) Production of live fish derived from frozen germ cells via germ cell transplantation. Stem Cell Res 29:103–110
Yoshizaki G, Yazawa R (2019) Application of surrogate broodstock technology in aquaculture. Fish Sci 85:429–437
Funding
This work was partly supported by JSPS KAKENHI Grant Number 23H00344 and JST-Mirai Program Grant Number JPMJMI21C1.
Author information
Authors and Affiliations
Contributions
K. S., A. Y., O. E., K. O., W. K., and R. Y. conducted experiments and analyzed the data. R. Y., Y. T., T. M., and G. Y. designed the research. R. Y. and G. Y. conceived the study and wrote the manuscript. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yazawa, R., Saitoh, K., Yamauchi, A. et al. Reproductive Characteristics and Suitability of Sterile dead end Knockout Nibe Croaker as a Recipient for Intraperitoneal Germ Cell Transplantation. Mar Biotechnol (2024). https://doi.org/10.1007/s10126-024-10323-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10126-024-10323-x