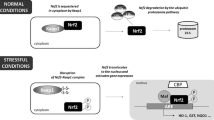
Abstract
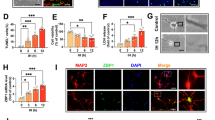
The ability of an animal to survive prolonged periods of oxygen deprivation is a critical area of study, both in terms of its importance to better understanding the physiology of these incredible animals and to its potential applicability to medical fields. The freshwater crayfish, Faxonius virilis, is one such animal capable of resisting anoxia, but it remains understudied and much of the metabolic mechanisms underlying this anoxia tolerance remain largely unprofiled. This study examines the activity and regulation of apoptosis and autophagy in F. virilis in response to 20-h anoxia. Apoptosis signaling was assessed through pro- and anti-apoptosis targets, whereas autophagy was assessed via expression response of multiple autophagy proteins. An anoxia-triggered, tissue-specific result arose, potentially based on the importance of individual organ integrity through hypometabolism. Tail muscle, which showed increased expression profiles of all three target groups, contrasted with hepatopancreas, which appeared to not be susceptible to either apoptotic or autophagic signaling during anoxia. This is likely due to the importance of the hepatopancreas, given that apoptosis or autophagy of this organ at any significant level could be fatal to the organism. The data provides a comprehensive overview of the responses and integration of multiple stress-responsive signaling pathways in F. virilis that provide a novel contribution to our understanding of pro-survival mechanisms supporting invertebrate anoxia resistance.







Similar content being viewed by others
References
Al-Attar R, Zhang Y, Storey KB (2017) Osmolyte regulation by TonEBP/NFAT5 during anoxia-recovery and dehydration- rehydration stresses in the freeze-tolerant wood frog (Rana sylvatica). PeerJ 2017(1). https://doi.org/10.7717/peerj.2797
Bai X, Jiang Y (2010) Key factors in mTOR regulation. In Cell Mol Life Sci 67:239–253
Bergamini E (2006) Autophagy: A cell repair mechanism that retards ageing and age-associated diseases and can be intensified pharmacologically. In Mol Asp Med 27:403–410
Biggar KK, Dubuc A, Storey K (2009) MicroRNA regulation below zero: Differential expression of miRNA-21 and miRNA-16 during freezing in wood frogs. Cryobiology 59:317–321
Blagosklonny MV (2001) Unwinding the loop of Bcl-2 phosphorylation. In Leukemia 15:869–874
Bonvillain CP, Rutherford DA, Kelso WE, Green CC (2012) Physiological biomarkers of hypoxic stress in red swamp crayfish Procambarus clarkii from field and laboratory experiments. Comparative Biochemistry and Physiology - A Molecular and Integrative Physiology 163:15–21
Bradford DF (1983) Winterkill, oxygen relations, and energy metabolism of a submerged dormant amphibian. Rana Muscosa Ecology 64:1171–1183
Brenner D, Mak TW (2009) Mitochondrial cell death effectors. In Curr Opin Cell Biol 21:871–877
Childers CL, Tessier SN, Storey KB (2019) The heart of a hibernator: EGFR and MAPK signaling in cardiac muscle during the hibernation of thirteen-lined ground squirrels, Ictidomys tridecemlineatus. PeerJ (9). https://doi.org/10.7717/peerj.7587
Cowan KJ, Storey KB (2001) Protein kinase and phosphatase responses to anoxia in crayfish, Orconectes virilis: Purification and characterization of cAMP-dependent protein kinase. Comparative Biochemistry and Physiology - B Biochemistry and Molecular Biology 130:565–577
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43:582–592
Dawson NJ, Storey KB (2011) Regulation of tail muscle arginine kinase by reversible phosphorylation in an anoxia-tolerant crayfish. J Comp Physiol [b]. https://doi.org/10.1007/s00360-011-0578-y
Dechend R, Hirano F, Lehmann K, Heissmeyer V, Ansicau S, Wulczyn FG, Scheidereit C, Leutz A (1999) The Bcl-3 oncoprotein acts as a bridging factor between NF-κB/Rel and nuclear co-regulators. Oncogene 18:3316–3323
Decuypere J-P, Parys JB, Bultynck G (2012) Regulation of the Autophagic Bcl-2/Beclin 1 Interaction. Cells 1:284–312
Deng X, Gao F, Flagg T, May WS (2004) Mono- and multisite phosphorylation enhances Bcl2’s antiapoptotic function and inhibition of cell cycle entry functions. Proc Natl Acad Sci USA 101:153–158
Deng X, Ruvolo P, Carr B, May WS (2000) Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc Natl Acad Sci USA 97:1578–1583
Deng X, Xiao L, Lang W, Gao F, Ruvolo P, May WS (2001) Novel Role for JNK as a Stress-activated Bcl2 Kinase. J Biol Chem 276:23681–23688
Díaz-Troya S, Pérez-Pérez ME, Florencio FJ, Crespo JL (2008) The role of TOR in autophagy regulation from yeast to plants and mammals. In Autophagy 4:851–865
Dohmen RJ, Huibregtse JM, Scheffner M (2016) Ubiquitin, Ubiquitin-Like Proteins, and Proteasome-Mediated Degradation. In Encyclopedia Cell Bio 1:582–595
Edlich F (2018) BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem Biophys Res Commun 500:26–34
English SG, Hadj-Moussa H, Storey KB (2018) MicroRNAs regulate survival in oxygen-deprived environments. J Exp Biol 221(23). https://doi.org/10.1242/jeb.190579
Eskelinen EL (2008) New Insights into the Mechanisms of Macroautophagy in Mammalian Cells. In Inter Rev Cell Mole Biol 266:207–247
Ferraro E, Cecconi F (2007) Autophagic and apoptotic response to stress signals in mammalian cells. In Arch Biochem Biophys 462:210–219
Gerber VEM, Wijenayake S, Storey KB (2016) Anti-apoptotic response during anoxia and recovery in a freeze-tolerant wood frog (Rana sylvatica). PeerJ. https://doi.org/10.7717/peerj.1834
Green SR, Storey KB (2016) Regulation of crayfish, Orconectes virilis, tail muscle lactate dehydrogenase (LDH) in response to anoxic conditions is associated with alterations in phosphorylation patterns. Comparative Biochemistry and Physiology Part - B: Biochemistry and Molecular Biology 202:67–74
Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, Inagaki F, Ohsumi Y (2007) The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem 282:37298–37302
He C, Levine B (2010) The Beclin 1 interactome. In Curr Opin Cell Biol 22:140–149
Hochachka PW, Lutz PL (2001) Mechanism, origin, and evolution of anoxia tolerance in animals. Comparative Biochemistry and Physiology - B Biochemistry and Molecular Biology 130:435–459
Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau VJ (1997) Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem 272:19022–19026
Huang WP, Scott SV, Kim J, Klionsky DJ (2000) The itinerary of a vesicle component, Aut7p/Cvt5p, terminates in the yeast vacuole via the autophagy/Cvt pathways. J Biol Chem 275:5845–5851
Ito T, Deng X, Carr B, May WS (1997) Bcl-2 phosphorylation required for anti-apoptosis function. J Biol Chem 272:11671–11673
Jhanwar-Uniyal M, Amin AG, Cooper JB, Das K, Schmidt MH, Murali R (2017) Discrete signaling mechanisms of mTORC1 and mTORC2: Connected yet apart in cellular and molecular aspects. In Adv Biol Regul 64:39–48
Jin M, Klionsky DJ (2014) Regulation of autophagy: Modulation of the size and number of autophagosomes. In FEBS Letters 588:2457–2463
Jung CH, Ro SH, Cao J, Otto NM, Kim DH (2010) MTOR regulation of autophagy. In FEBS Letters 584:1287–1295
Kaczanowski S (2016) Apoptosis: Its origin, history, maintenance and the medical implications for cancer and aging. In Phys Biol 13(3). https://doi.org/10.1088/1478-3975/13/3/031001
Kamada Y, Yoshino K, Kondo C, Kawamata T, Oshiro N, Yonezawa K, Ohsumi Y (2010) Tor Directly Controls the Atg1 Kinase Complex To Regulate Autophagy. Mol Cell Biol 30:1049–1058
Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. In Cell Death Differ 18:571–580
Kerr JFR, Wyllie AH, Currie AR (1972) Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Klionsky DJ, Eskelinen EL, Deretic V (2014) Autophagosomes, phagosomes, autolysosomes, phagolysosomes, autophagolysosomes... Wait, I’m confused. In Autophagy 10:549–551
Kønig SM, Rissler V, Terkelsen T, Lambrughi M, Papaleo E (2019) Alterations of the interactome of Bcl-2 proteins in breast cancer at the transcriptional, mutational and structural level. PLoS Comput Biol 15(12). https://doi.org/10.1371/journal.pcbi.1007485
Levine B, Sinha S, Kroemer G (2008) Bcl-2 family members: Dual regulators of apoptosis and autophagy. In Autophagy 4:600–606
Li M, Fu Y, Yang Z, Yin XM (2017) Measurement of the Activity of the Atg4 Cysteine Proteases. In Meth Enzymol 587:207–225
Lishner M, Lalkin A, Klein A, Yarkoni S, Manor Y, Fejgin M, Leytin V, Ravid M, Amiel A (1995) The BCL-1, BCL-2, and BCL-3 oncogenes are involved in chronic lymphocytic leukemia. Detection by fluorescence in situ hybridization. Cancer Genetics and Cytogenetics 85:118–123
Logan SM, Luu BE, Storey KB (2016) Turn down genes for WAT? Activation of anti-apoptosis pathways protects white adipose tissue in metabolically depressed thirteen-lined ground squirrels. Mol Cell Biochem 416:47–62
Lv D, Guo L, Zhang T, Huang L (2017) PRAS40 signaling in tumor. Oncotarget 8:69076–69085
Mattice AMS, MacLean IA, Childers CL, Storey KB (2018) Characterization of pyruvate kinase from the anoxia tolerant turtle. Trachemys scripta elegans: A potential role for enzyme methylation during metabolic rate depression. PeerJ (6). https://doi.org/10.7717/peerj.4918
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. In Nature 451:1069–1075
Mörck C, Pilon M (2007) Caloric restriction and autophagy in Caenorhabditis elegans. In Autophagy 3:51–53
Morin PJ, Dubuc A, Storey KB (2008) Differential expression of microRNA species in organs of hibernating ground squirrels: A role in translational suppression during torpor. Biochimica Et Biophysica Acta - Gene Regulatory Mechanisms 1779:628–633
Nobukuni T, Kozma SC, Thomas G (2007) hvps34, an ancient player, enters a growing game: mTOR Complex1/S6K1 signaling. In Curr Opin Cell Biol 19:135–141
Noda T (2017) Regulation of Autophagy through TORC1 and mTORC1. Biomolecules 7(3). https://doi.org/10.3390/biom7030052
Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P (2008) Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie 90:313–323
Pimkina J, Humbey O, Zilfou JT, Jarnik M, Murphy ME (2009) ARF induces autophagy by virtue of interaction with Bcl-xl. J Biol Chem 284:2803–2810
Poveda J, Sanz AB, Carrasco S, Ruiz-Ortega M, Cannata-Ortiz P, Sanchez-Niño MD, Ortiz A (2017) Bcl3: A regulator of NF-κB inducible by TWEAK in acute kidney injury with anti-inflammatory and antiapoptotic properties in tubular cells. Exp Mol Med 49(7). https://doi.org/10.1038/emm.2017.89
Puente C, Hendrickson RC, Jiang X (2016) Nutrient-regulated phosphorylation of ATG13 inhibits starvation-induced autophagy. J Biol Chem 291:6026–6035
Reiber CL (1995) Physiological adaptations of crayfish to the hypoxic environment. Integr Comp Biol 35:1–11
Rouble AN, Hefler J, Mamady H, Storey KB, Tessier SN (2013) Anti-apoptotic signaling as a cytoprotective mechanism in mammalian hibernation. PeerJ (1). https://doi.org/10.7717/peerj.29
Ruoff R, Katsara O, Kolupaeva V (2016) Cell type-specific control of protein synthesis and proliferation by FGF-dependent signaling to the translation repressor 4E-BP. Proc Natl Acad Sci USA 113:7545–7550
Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM (2007) PRAS40 Is an Insulin-Regulated Inhibitor of the mTORC1 Protein Kinase. Mol Cell 25:903–915
Saxton RA, Sabatini DM (2017) mTOR Signaling in Growth, Metabolism, and Disease. In Cell 168:960–976
Seibel BA, Luu BE, Tessier SN, Towanda T, Storey KB (2018) Metabolic suppression in the pelagic crab, Pleuroncodes planipes, in oxygen minimum zones. Comparative Biochemistry and Physiology Part - B: Biochemistry and Molecular Biology 224:88–97
Shpilka T, Weidberg H, Pietrokovski S, Elazar Z (2011) Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol 12(7). https://doi.org/10.1186/gb-2011-12-7-226
Smith DJ, Ng H, Kluck RM, Nagley P (2008) The mitochondrial gateway to cell death. In IUBMB Life 60:383–389
Storey KB (2015) Regulation of hypometabolism: Insights into epigenetic controls. In J Exp Biol 218:150–159
Swiech L, Perycz M, Malik A, Jaworski J (2008) Role of mTOR in physiology and pathology of the nervous system. In Biochimica et Biophysica Acta - Proteins and Proteomics 1784:116–132
Szereszewski KE, Storey KB (2018) Translational regulation in the anoxic turtle, Trachemys scripta elegans. Mol Cell Biochem 445:13–23
Tanigawa M, Maeda T (2017) An In Vitro TORC1 Kinase Assay That Recapitulates the Gtr-Independent Glutamine-Responsive TORC1 Activation Mechanism on Yeast Vacuoles. Mole Cell Biol 37(14). https://doi.org/10.1128/mcb.00075-17
Walczak M, Martens S (2013) Dissecting the role of the Atg12-Atg5-Atg16 complex during autophagosome formation. Autophagy 9:424–425
Wang L, Harris TE, Roth RA, Lawrence JC (2007) PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem 282:20036–20044
Wang T, Edgar BA (2010) TOR signaling and cell death. In Enzymes 28(C). https://doi.org/10.1016/S1874-6047(10)28011-3
Wei Y, Sinha S, Levine B (2008) Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy 4:949–951
Wu CW, Storey KB (2012) Regulation of the mTOR signaling network in hibernating thirteen-lined ground squirrels. J Exp Biol 215:1720–1727
Xiao L, Wang YC, Li WS, Du Y (2009) The role of mTOR and phospho-p70S6K in pathogenesis and progression of gastric carcinomas: An immunohistochemical study on tissue microarray. J Exp Clin Cancer Res 28(1). https://doi.org/10.1186/1756-9966-28-152
Yorimitsu T, Klionsky DJ (2005) Autophagy: Molecular machinery for self-eating. In Cell Death Diff 12:1542–1552
Zhang J, Storey KB (2016) RBioplot: An easy-to-use R pipeline for automated statistical analysis and data visualization in molecular biology and biochemistry. PeerJ (9). https://doi.org/10.7717/peerj.2436
Zhang KS, Schecker J, Krull A, Riechert E, Jürgensen L, Kamuf-Schenk V, Burghaus J, Kiper L, Cao Ho T, Wöltje K, Stangl V, Katus HA, Stangl K, Völkers M, Althoff TF (2019) PRAS40 suppresses atherogenesis through inhibition of mTORC1-dependent pro-inflammatory signaling in endothelial cells. Scien Rep 9(1). https://doi.org/10.1038/s41598-019-53098-1
Ziello JE, Jovin IS, Huang Y (2007) Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. In Yale J Biol Med 80(2):51–60
Acknowledgements
The authors thank Benjamin Lant for his experimental contributions.
Funding
This work was supported by a Discovery grant (Grant # 6793) from the Natural Sciences and Engineering Research Council (NSERC) of Canada. KBS holds the Canada Research Chair in Molecular Physiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no competing interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Breedon, S.A., Gupta, A. & Storey, K.B. Regulation of Apoptosis and Autophagy During Anoxia in the Freshwater Crayfish, Faxonius virilis. Mar Biotechnol 24, 626–639 (2022). https://doi.org/10.1007/s10126-022-10132-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-022-10132-0