Abstract
Background
Advanced gastric cancer (GC) has a poor prognosis. This study aimed to identify novel GC-related genes as potential therapeutic targets.
Methods
Killer cell lectin-like receptor G2 (KLRG2) was identified as a candidate gene by transcriptome analysis of metastatic GC tissues. Small interfering RNA-mediated KLRG2 knockdown in human GC cell lines was used to investigate KLRG2 involvement in signaling pathways and functional behaviors in vitro and in vivo. Clinicopathological data were analyzed in patients stratified according to tumor KLRG2 mRNA expression.
Results
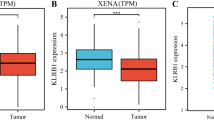
KLRG2 knockdown in GC cells decreased cell proliferation, migration, and invasion; caused cell cycle arrest in G2/M phase; induced apoptosis via caspase activation; suppressed JAK/STAT and MAPK-ERK1/2 pathway activities; and upregulated p53 and p38 MAPK activities. In mouse xenograft models of peritoneal metastasis, the number and weight of disseminated GC nodules were decreased by KLRG2 knockdown. High tumor levels of KLRG2 mRNA were significantly associated with lower 5-year overall survival (OS) and relapse-free survival (RFS) rates in patients with Stage I–III GC (5-year OS rate: 64.4% vs. 80.0%, P = 0.009; 5-year RFS rate: 62.8% vs. 78.1%, P = 0.030).
Conclusions
KLRG2 knockdown attenuated the malignant phenotypes of GC cells via downregulation of JAK/STAT and MAPK-ERK1/2 pathway activity and upregulation of p38 MAPK and p53. Targeted suppression of KLRG2 may serve as a new treatment approach for GC.






Similar content being viewed by others
References
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–48. https://doi.org/10.1016/s0140-6736(20)31288-5.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Suzuki S, Takahashi A, Ishikawa T, Akazawa K, Katai H, Isobe Y, et al. Surgically treated gastric cancer in Japan: 2011 annual report of the national clinical database gastric cancer registry. Gastric Cancer. 2021;24(3):545–66. https://doi.org/10.1007/s10120-021-01178-5.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. https://doi.org/10.1007/s10120-016-0622-4.
Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–84. https://doi.org/10.1007/s10120-014-0402-y.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71. https://doi.org/10.1016/s0140-6736(17)31827-5.
Kanda M, Kasahara Y, Shimizu D, Miwa T, Umeda S, Sawaki K, et al. Amido-bridged nucleic acid-modified antisense oligonucleotides targeting SYT13 to treat peritoneal metastasis of gastric cancer. Mol Ther Nucleic Acids. 2020;22:791–802. https://doi.org/10.1016/j.omtn.2020.10.001.
Kanda M, Shimizu D, Sawaki K, Nakamura S, Umeda S, Miwa T, et al. Therapeutic monoclonal antibody targeting of neuronal pentraxin receptor to control metastasis in gastric cancer. Mol Cancer. 2020;19(1):131. https://doi.org/10.1186/s12943-020-01251-0.
Kanda M, Suh YS, Park DJ, Tanaka C, Ahn SN, Kong SH, et al. Serum levels of ANOS1 serve as a diagnostic biomarker of gastric cancer: a prospective multicenter observational study. Gastric Cancer. 2020;23(2):203–11. https://doi.org/10.1007/s10120-019-00995-z.
Kanda M, Shimizu D, Nakamura S, Sawaki K, Umeda S, Miwa T, et al. Blockade of CHRNB2 signaling with a therapeutic monoclonal antibody attenuates the aggressiveness of gastric cancer cells. Oncogene. 2021;40(36):5495–504. https://doi.org/10.1038/s41388-021-01945-9.
Liu X, Cheng I, Plummer SJ, Suarez BK, Casey G, Catalona WJ, et al. Fine-mapping of prostate cancer aggressiveness loci on chromosome 7q22-35. Prostate. 2011;71(7):682–9. https://doi.org/10.1002/pros.21284.
Wang XW, Gao J, Xu YH, Xu JD, Fan ZX, Zhao XF, et al. Novel pattern recognition receptor protects shrimp by preventing bacterial colonization and promoting phagocytosis. J Immunol. 2017;198(8):3045–57. https://doi.org/10.4049/jimmunol.1602002.
Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–31. https://doi.org/10.1016/s0092-8674(00)81871-1.
Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12(19):2973–83. https://doi.org/10.1101/gad.12.19.2973.
Hu W, Feng Z, Levine AJ. The regulation of multiple p53 stress responses is mediated through MDM2. Genes Cancer. 2012;3(3–4):199–208. https://doi.org/10.1177/1947601912454734.
Koul HK, Pal M, Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer. 2013;4(9–10):342–59. https://doi.org/10.1177/1947601913507951.
Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501. https://doi.org/10.1016/s0092-8674(00)81590-1.
Eblen ST. Extracellular-regulated kinases: signaling from Ras to ERK substrates to control biological outcomes. Adv Cancer Res. 2018;138:99–142. https://doi.org/10.1016/bs.acr.2018.02.004.
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007. https://doi.org/10.3892/etm.2020.8454.
Bolli R, Dawn B, Xuan YT. Role of the JAK-STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends Cardiovasc Med. 2003;13(2):72–9. https://doi.org/10.1016/s1050-1738(02)00230-x.
Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32(21):2601–13. https://doi.org/10.1038/onc.2012.347.
Mondal S, Adhikari N, Banerjee S, Amin SA, Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: a minireview. Eur J Med Chem. 2020;194:112260. https://doi.org/10.1016/j.ejmech.2020.112260.
Liu Y, Liu H, Luo X, Deng J, Pan Y, Liang H. Overexpression of SMYD3 and matrix metalloproteinase-9 are associated with poor prognosis of patients with gastric cancer. Tumour Biol. 2015;36(6):4377–86. https://doi.org/10.1007/s13277-015-3077-z.
Kolegraff K, Nava P, Helms MN, Parkos CA, Nusrat A. Loss of desmocollin-2 confers a tumorigenic phenotype to colonic epithelial cells through activation of Akt/β-catenin signaling. Mol Biol Cell. 2011;22(8):1121–34. https://doi.org/10.1091/mbc.E10-10-0845.
Fang WK, Liao LD, Li LY, Xie YM, Xu XE, Zhao WJ, et al. Down-regulated desmocollin-2 promotes cell aggressiveness through redistributing adherens junctions and activating beta-catenin signalling in oesophageal squamous cell carcinoma. J Pathol. 2013;231(2):257–70. https://doi.org/10.1002/path.4236.
Yang M, Gu YY, Peng H, Zhao M, Wang J, Huang SK, et al. NAIF1 inhibits gastric cancer cells migration and invasion via the MAPK pathways. J Cancer Res Clin Oncol. 2015;141(6):1037–47. https://doi.org/10.1007/s00432-014-1865-2.
Wei XD, Liu X, Liu HM, He X, Zhuang H, Tang YP, et al. BRCA1-associated protein induced proliferation and migration of gastric cancer cells through MAPK pathway. Surg Oncol. 2020;35:191–9. https://doi.org/10.1016/j.suronc.2020.08.007.
Jia SQ, Lu JJ, Qu TT, Feng Y, Wang XH, Liu CX, et al. MAGI1 inhibits migration and invasion via blocking MAPK/ERK signaling pathway in gastric cancer. Chin J Cancer Res. 2017;29(1):25–35. https://doi.org/10.21147/j.issn.1000-9604.2017.01.04.
Shi Y, Sun HH. Down-regulation of lncRNA LINC00152 suppresses gastric cancer cell migration and invasion through inhibition of the ERK/MAPK signaling pathway. Onco Targets Ther. 2020;13:2115–24. https://doi.org/10.2147/ott.s217452.
Maeda M, Taniguchi H, Sekiguchi S, Katai H, Kushima R. Adenosquamous cell carcinoma of the stomach-a clinicopathologic analysis of 23 cases. Stomach Intestine (abstract in English). 2010;45(12):1959–66. https://doi.org/10.11477/mf.1403102164.
Acknowledgements
We thank Anne M. O’Rourke, PhD, from Edanz (www.edanz.com/ac) for editing a draft of this manuscript.
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethics approval and consent to participate
This study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki (2013) Ethical Principles for Medical Research Involving Human Subjects and was approved by the Institutional Review Board of Nagoya University (approval no. 2014-0043). Written informed consent was obtained from all patients for the use of clinical samples and data. The Animal Research Committee of Nagoya University approved the experiments using animals (approval no. 28210).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10120_2024_1480_MOESM1_ESM.jpg
Supplementary file1 Supplementary Fig. 1. Knockdown efficiency of KLRG2 siRNA by RT-qPCR on Day 0, Day 2, Day 4, and Day 6 (JPG 3515 KB)
10120_2024_1480_MOESM2_ESM.jpg
Supplementary file2 Supplementary Fig. 2. Expression level of proteins involved in intracellular signaling pathways evaluated by Simple Western assays. a JAK/STAT pathway. b p53. c Cyclins. d p38 MAPK pathway. e ERK1/2 pathway (JPG 5015 KB)
10120_2024_1480_MOESM3_ESM.jpg
Supplementary file3 Supplementary Fig. 3. Immunofluorescence microscopy of MKN1 labeled with KLRG2-Ab (green), Actin (red), and DAPI (blue) (JPG 2684 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ito, Y., Kanda, M., Sasahara, M. et al. Killer cell lectin-like receptor G2 facilitates aggressive phenotypes of gastric cancer cells via dual activation of the ERK1/2 and JAK/STAT pathways. Gastric Cancer 27, 506–518 (2024). https://doi.org/10.1007/s10120-024-01480-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-024-01480-y