Abstract
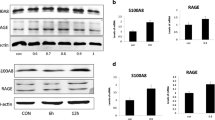
In recent years, some treatments for esthetic and pathologic skin conditions have increasingly been based on the use of non-ablative neodymium-doped yttrium aluminum garnet (Nd:YAG) laser due to its greater penetration ability than other types of lasers, few contraindications, minimal side effects, no damage for epidermidis and the rapid recovery of the treated patients. The skin is frequently exposed to many stressors such as radiation, toxic substances, metabolites, foods, mechanical insults, and allergen exposition that cause oxidative damage and have a decisive influence on the aging process. The imbalance between reactive oxygen species, reactive nitrogen species, and the malfunctioning of the antioxidant defense system promotes the establishment of an excessive inflammatory process, which can induce various diseases including cancer and neurodegenerative disorders. The present study investigated the cytoprotective function of Q-switched Nd:YAG laser against stress aging and cell injury in HaCaT cells. We evaluated the effect of the laser on antioxidant defenses, inflammation, metalloproteinases’ expression, and the AhR-Nrf2 pathway. Q-switched Nd:YAG is able to upregulate the AhR pathway and the expression of IL-6 and TGF-β, which are involved in wound repair process, and to downregulate the expression of MMP-2 and 9, so preventing the collagen degradation. Q-switched Nd:YAG can stimulate the cellular antioxidant defenses by activating the AhR-Nrf2 system.



Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Jacques SL (1992) Laser-tissue interactions: photochemical, photothermal, and photomechanical. Surg Clin North Am 72(3):531–558. https://doi.org/10.1016/S0039-6109(16)45731-2.ISSN0039-6109
Roh MR, Goo BC, Jung JY, Chung HJ, Chung KY (2011) Treatment of enlarged pores with the quasi long-pulsed versus Q-switched 1064 nm Nd:YAG lasers: a split-face, comparative, controlled study. Laser Ther 20(3):175–180
Kim JM, Kim WI, Ko HC, Kim MB, Kim BS (2017) Epidermal barrier function changes after ablative and non-ablative fractional laser administration. J Eur Acad Dermatol Venereol 31(2):e83–e85
Nisticò SP, Cannarozzo G, Provenzano E et al (2021) Nanosecond Q-switched 1064/532 nm laser to treat hyperpigmentations: a double center retrospective study. Clin Pract 11(4):708–714. https://doi.org/10.3390/clinpract11040086. Published 2021 Sep 23
Mani N, Zorman A (2021) Acne scar treatment using high-energy fractional nanosecond Q-switched 1064 nm laser. J Cosmet Dermatol 20:3907–3912. https://doi.org/10.1111/jocd.14534
Papadelli P, Kyriakidou K, Kotsakis GA, Pepelassi E, Kallis A, Vrotsos IA, Karoussis IK (2021) Immunomodulatory effects of Nd:YAG (1064 nm) and diode laser (810 nm) wavelengths to LPS-challenged human gingival fibroblasts. Arch Oral Bio 122:104982. https://doi.org/10.1016/j.archoralbio.2020.104982.ISSN0003-9969
Shiba H, Tsuda H, Kajiya M, Fujita T, Takeda K, Hino T, Kawaguchi H, Kurihara H (2009) Neodymium-doped yttrium-aluminium-garnet laser irradiation abolishes the increase in interleukin-6 levels caused by peptidoglycan through the p38 mitogen-activated protein kinase pathway in human pulp cells. J Endod 35(3):373–376. https://doi.org/10.1016/j.joen.2008.11.028.ISSN0099-2399
Kim K, Kim IS, Cho TH, Seo YK, Hwang SJ (2015) High-intensity Nd:YAG laser accelerates bone regeneration in calvarial defect models. J Tissue Eng Regen Med 9(8):943–951. https://doi.org/10.1002/term.1845
Ye X, Wang L, Dang Y, Liu B, Zhao D (2013) Investigation of the 1064nm Q-switched Nd:YAG laser on collagen expression in an animal model. Photomed Laser Surg 30(10):604–609. https://doi.org/10.1089/pho.2012.3221
Polnikorn N (2008) Treatment of refractory dermal melasma with the MedLite C6 Q-switched Nd:YAG laser: two case reports. J Cosmet Laser Ther 10(3):167–173. https://doi.org/10.1080/14764170802179687
Friedman PM, Jih MH, Skover GR, Payonk GS, Kimyai-Asadi A, Geronemus RG (2004) Treatment of atrophic facial acne scars with the 1064-nm Q-switched Nd:YAG laser: six-month follow-up study. Arch Dermatol 140(11):1337–1341. https://doi.org/10.1001/archderm.140.11.1337
Har-Shai Y, Mettanes I, Zilberstein Y, Genin O, Spector I, Pines M (2011) Keloid histopathology after intralesional cryosurgery treatment. J Eur Acad Dermatol Venereol 25(9):1027–1036. https://doi.org/10.1111/j.1468-3083.2010.03911.x
Nanni CA, Alster TS (1997) Optimizing treatment parameters for hair removal using a topical carbon-based solution and 1064-nm Q-switched neodymium:YAG laser energy. Arch Dermatol 133(12):1546–1549
Griffith RD, Simmons BJ, Bray FN, Falto-Aizpurua LA, Yazdani Abyaneh MA, Nouri K (2015) 1064 nm Q-switched Nd:YAG laser for the treatment of Argyria: a systematic review. J Eur Acad Dermatol Venereol 29(11):2100–2103. https://doi.org/10.1111/jdv.13117
Cho SB, Lee JH, Lee SH, Lee SJ, Bang D, Oh SH (2010) Efficacy and safety of 1064-nm Q-switched Nd:YAG laser with low fluence for keloids and hypertrophic scars. J Eur Acad Dermatol Venereol 24(9):1070–1074. https://doi.org/10.1111/j.1468-3083.2010.03593.x
Bhatta AK, Huang X, Keyal U, Zhao JJ (2014) Laser treatment for onychomycosis: a review. Mycoses 57(12). https://doi.org/10.1111/myc.12225
Baroni A, De Filippis A, Oliviero G, Fusco A, Perfetto B, Buommino E, Donnarumma G (2018) Effect of 1064-nm Q-switched Nd:YAG laser on invasiveness and innate immune response in keratinocytes infected with Candida albicans. Lasers Med Sci 33:941–948. https://doi.org/10.1007/s10103-017-2407-3
Fusco A, Savio V, Cammarota M, Donnarumma G, Baroni A (2021) Decreased expression of Malassezia furfur virulence factors after Q-switched Nd:YAG laser irradiation. Eur J Dermatol. https://doi.org/10.1684/ejd.2021.4106
Liguori I, Russo G, Curcio F et al (2018) Oxidative stress, aging, and diseases. Clin Interv Aging. 13:757–772. https://doi.org/10.2147/CIA.S158513. Published 2018 Apr 26
Parrado C, Mercado-Saenz S, Perez-Davo A, Gilaberte Y, Gonzalez S, Juarranz A (2019) Environmental stressors on skin aging. Mechanistic insights. Front Pharmacol 10:759. https://doi.org/10.3389/fphar.2019.00759. Published 2019 Jul 9
Heck DE, Vetrano AM, Mariano TM, Laskin JD (2003) UVB light stimulates production of reactive oxygen species. Unexpected role for catalase. J Biol Chem 278(25):22432–22436
Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E (2021) The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci 22(9):4642. https://doi.org/10.3390/ijms22094642. Published 2021 Apr 28
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta Mol Cell Res 1863(12):2977–2992. https://doi.org/10.1016/j.bbamcr.2016.09.012.ISSN0167-4889
Yamamoto Y (2001) Role of active oxygen species and antioxidants in photoaging. J Dermatol Sci 27(Suppl. 1):1–4
Forcados GE, Muhammad A, Oladipo OO, Makama S, Meseko CA (2021) Metabolic implications of oxidative stress and inflammatory process in SARS-CoV-2 pathogenesis: therapeutic potential of natural antioxidants. Front Cell Infect Microbiol 2021(11):654813. https://doi.org/10.3389/fcimb.2021.654813
Srivastava KK, Kumar R (2015) Stress, oxidative injury and disease. Indian J Clin Biochem 30:3–10. https://doi.org/10.1007/s12291-014-0441-5
Checa J, Aran JM (2020) Reactive oxygen species: drivers of physiological and pathological processes. J Inflamm Res 13:1057–1073. https://doi.org/10.2147/JIR.S275595
Furue M, Takahara M, Nakahara T, Uchi H (2014) Role of AhR/ARNT system in skin homeostasis. Arch Dermatol Res 306(9):769–779. https://doi.org/10.1007/s00403-014-1481-7
Buommino E, Baroni A, Papulino C, Nocera FP, Coretti L, Donnarumma G, De Filippis A, De Martino L (2018) Malassezia pachydermatis up-regulates AhR related CYP1A1 gene and epidermal barrier markers in human keratinocytes. Med Mycol 56(8):987–993. https://doi.org/10.1093/mmy/myy004
Moorthy B, Chu C, Carlin DJ (2015) Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci 145(1):5–15. https://doi.org/10.1093/toxsci/kfv040
Tonolo F, Moretto L, Grinzato A et al (2020) Fermented soy-derived bioactive peptides selected by a molecular docking approach show antioxidant properties involving the Keap1/Nrf2 Pathway. Antioxidants (Basel) 9(12):1306. https://doi.org/10.3390/antiox9121306. Published 2020 Dec 19
Kim M, Jee SC, Kim KS, Kim HS, Yu KN, Sung JS (2021) Quercetin and isorhamnetin attenuate benzo[a]pyrene-induced toxicity by modulating detoxification enzymes through the AhR and NRF2 signaling pathways. Antioxidants (Basel) 10(5):787. https://doi.org/10.3390/antiox10050787. Published 2021 May 16.
Takei K, Hashimoto-Hachiya A, Takahara M, Tsuji G, Nakahara T, Furue M (2015) Cynaropicrin attenuates UVB-induced oxidative stress via the AhR–Nrf2–Nqo1 pathway. Toxicol Lett 234(2):74–80. https://doi.org/10.1016/j.toxlet.2015.02.007.ISSN0378-4274
Tsuji G, Takahara M, Uchi H, Matsuda T, Chiba T, Takeuchi S, Yasukawa F, Moroi Y, Furue M (2012) Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: the basis of its anti-inflammatory effect. J Investig Dermatol 132(1):59–68
Buelna-Chontal M, Zazueta C (2013) Redox activation of Nrf2 & NF-κB: a double end sword? Cell Signal 25(12):2548–2557. https://doi.org/10.1016/j.cellsig.2013.08.007
Lee J-M, Johnson JA (2004) An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol 37(2):139–143. https://doi.org/10.5483/bmbrep.2004.37.2.139
Hiebert P (2021) The Nrf2 transcription factor: a multifaceted regulator of the extracellular matrix. Matrix Biol Plus 10:100057. https://doi.org/10.1016/j.mbplus.2021.100057. Published 2021 Feb 20
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13(1):76–86
Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M (2004) Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24(16):7130–7139. https://doi.org/10.1128/MCB.24.16.7130-7139.2004
Wasik U, Milkiewicz M, Kempinska-Podhorodecka A, Milkiewicz P (2017) Protection against oxidative stress mediated by the Nrf2/Keap1 axis is impaired in Primary Biliary Cholangitis. Sci Rep 7:44769. https://doi.org/10.1038/srep44769. Published 2017 Mar 23
Jung KA, Kwak MK (2010) The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 15(10):7266-7291. https://doi.org/10.3390/molecules15107266. Published 2010 Oct 20
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Fusco A, Coretti L, Savio V, Buommino E, Lembo F Donnarumma G (2017) Biofilm formation and immunomodulatory activity of Proteus mirabilis clinically isolated strains. Int J Mol Sci 18:414
Paoletti I, Fusco A, Grimaldi E, Perillo L, Coretti L, Di Domenico M, Cozza V, Contaldo M, Serpico R, Guida A, Donnarumma G (2013) Assessment of host defence mechanisms induced by Candida species. Int J Immunopathol Pharmacol 26(3):663–672. https://doi.org/10.1177/039463201302600309
Sorg O, Zennegg M, Schmid P, Fedosyuk R, Valikhnovskyi R, Gaide O, Kniazevych V, Saurat JH (2009) 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) poisoning in Victor Yushchenko: identification and measurement of TCDD metabolites. Lancet 374(9696):1179–1185. https://doi.org/10.1016/S0140-6736(09)60912-0
Furue M, Uchi H, Mitoma C, Hashimoto-Hachiya A, Chiba T, Ito T, Nakahara T, Tsuji G (2017) Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factor-erythroid 2-related factor-2. Nutrients 9(3):223. https://doi.org/10.3390/nu9030223
Uchi H, Yasumatsu M, Morino-Koga S, Mitoma C, Furue M (2017) Inhibition of aryl hydrocarbon receptor signaling and induction of NRF2-mediated antioxidant activity by cinnamaldehyde in human keratinocytes. J Dermatol Sci 85(1):36–43. https://doi.org/10.1016/j.jdermsci.2016.10.003
Fuyuno Y, Uchi H, Yasumatsu M et al (2018) Perillaldehyde inhibits AHR signaling and activates NRF2 antioxidant pathway in human keratinocytes [published correction appears in Oxid Med Cell Longev. 2018 May 22;2018:6091947]. Oxid Med Cell Longev 2018:9524657. https://doi.org/10.1155/2018/9524657. Published 2018 Feb 14
Nakahara T, Mitoma C, Hashimoto-Hachiya A, Takahara M, Tsuji G, Uchi H, Yan X, Hachisuka J, Chiba T, Esaki H, Kido-Nakahara M, Furue M (2015) Antioxidant Opuntia ficus-indica extract activates AHR-NRF2 signaling and upregulates filaggrin and loricrin expression in human keratinocytes. J Med Food 18(10):1143–1149. https://doi.org/10.1089/jmf.2014.3396
Saw CL, Huang MT, Liu Y, Khor TO, Conney AH, Kong AN (2011) Impact of Nrf2 on UVB-induced skin inflammation/photoprotection and photoprotective effect of sulforaphane. Mol Carcinog 50(6):479–486. https://doi.org/10.1002/mc.20725
Haarmann-Stemmann T, Esser C, Krutmann J (2015) The Janus-faced role of aryl hydrocarbon receptor (AHR) signaling in the skin: consequences for prevention and treatment of skin disorders. J Invest Dermatol 135(11):2572–2576
Slutsky JB, Clark RA, Remedios AA, Klein PA (2010) An evidence-based review of the efficacy of coal tar preparations in the treatment of psoriasis and atopic dermatitis. J Drugs Dermatol 9(10):1258–1264
Corazza M, Odorici G, Conti A, Di Lernia V, Motolese A, Bardazzi F, Di Nuzzo S, Monti A, Arginelli F, Filippi F, Valpiani G, Morotti C, Borghi A (2021) Dimethyl fumarate treatment for psoriasis in a real-life setting: a multicentric retrospective study. Dermatol Ther 34(5):e15066. https://doi.org/10.1111/dth.15066
Biolcati G, Aurizi C, Barbieri L, Cialfi S, Screpanti I, Talora C (2013) Efficacy of the melanocortin analogue Nle4-D-Phe7-α-melanocyte-stimulating hormone in the treatment of patients with Hailey-Hailey disease. Clin Exp Dermatol 39:168–175
Lin X, Meng X, Song Z, Lin J (2020) Nuclear factor erythroid 2-related factor 2 (Nrf2) as a potential therapeutic target for vitiligo. Arch Biochem Biophys 696:108670. https://doi.org/10.1016/j.abb.2020.108670
Liu B, Su K, Wang J, Wang J, Xin Z, Li F, Fu Y (2018) Corynoline exhibits anti-inflammatory effects in lipopolysaccharide (LPS)-stimulated human umbilical vein endothelial cells through activating Nrf2. Inflammation 41(5):1640–1647. https://doi.org/10.1007/s10753-018-0807-6
Rumalla VK, Borah GL (2001) Cytokines, growth factors, and plastic surgery. Plast Reconstr Surg 108(3):719–733. https://doi.org/10.1097/00006534-200109010-00019
De Filippis A, Perfetto B, Guerrera LP, Oliviero G, Baroni A (2019) Q-switched 1064 nm Nd-Yag nanosecond laser effects on skin barrier function and on molecular rejuvenation markers in keratinocyte-fibroblasts interaction. Lasers Med Sci 34(3):595–605. https://doi.org/10.1007/s10103-018-2635-1
de Filippis A, D’Agostino A, Pirozzi AVA, Tufano MA, Schiraldi C, Baroni A (2021) Q-switched Nd-YAG laser alone and in combination with innovative hyaluronic acid gels improve keratinocytes wound healing in vitro. Lasers Med Sci 36(5):1047–1057. https://doi.org/10.1007/s10103-020-03145-5
Jansen PL, Rosch R, Jansen M, Binnebösel M, Junge K, Alfonso-Jaume A, Klinge U, Lovett DH, Mertens PR (2007) Regulation of MMP-2 gene transcription in dermal wounds. J Investig Dermatol 127(7):1762–1767. https://doi.org/10.1038/sj.jid.5700765
Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, Varani J, Kang S, Voorhees JJ (2009) Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol 174(1):101–114. https://doi.org/10.2353/ajpath.2009.080599
Hong SE, Hong MK, Kang SR, Young PB (2016) Effects of neodymium-yttrium-aluminum garnet (Nd:YAG) pulsed high-intensity laser therapy on full thickness wound healing in an experimental animal model. J Cosmet Laser Ther 18(8):432–437. https://doi.org/10.1080/14764172.2016.1202421
Author information
Authors and Affiliations
Contributions
AB and GD designed the study. AF, BP, VS, and MD oversaw the laboratory procedures. AF wrote the manuscript. AB, ES, and GD supervised and validated the original draft. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fusco, A., Savio, V., Perfetto, B. et al. Q-switched Nd:YAG laser protects human keratinocytes from oxidative stress and inflammation via AhR–Nrf2 pathway. Lasers Med Sci 39, 7 (2024). https://doi.org/10.1007/s10103-023-03953-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-023-03953-5