Abstract
Purpose
Cancer patients are at heightened risk for invasive aspergillosis (IA), a condition associated with elevated mortality risk. The JF5-based Aspergillus Galactomannoprotein Lateral Flow Device (AspLFD) offers rapid point-of-care testing (POCT) for IA. This study evaluated the diagnostic performance of AspLFD in cancer populations.
Methods
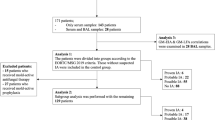
This retrospective study examined cancer patient bronchoalveolar lavage fluid (BALF) and serum samples collected between September 2021 and January 2023. Both AspLFD and galactomannan (GM) assays were conducted, and the results were analysed by two independent researchers.
Results
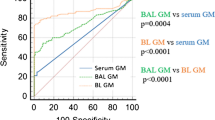
This study included 242 samples from 218 cancer patients, with 58 BALF and 184 serum samples. The overall agreement between AspLFD and GM assay results was 92.1%, with a kappa value of 0.552. AspLFD diagnosed proven/probable IA with a sensitivity and specificity of 91.7% and 95.3%, respectively, whereas GM exhibited sensitivity and specificity values of 83.3% and 93.7%, respectively. There were no statistical differences in the sensitivity and specificity between the two methods (P > 0.05). For serum analyses, AspLFD and GM exhibited similar sensitivity (66.7% vs. 66.7%, P > 0.05) and specificity (98.6% vs. 96.6%, P > 0.05) values. However, the sensitivity of the AspLFD was superior to the GM assay (100% vs. 88.9%) in BALF analyses but the difference was not statistically significant (P > 0.05), with no difference in specificity (83.7% vs. 83.7%, P > 0.05). In the solid-tumour cohort, both the AspLFD and GM assay exhibited high sensitivity (100% for both) and specificity (94.2% vs. 92.8%, P > 0.05).
Conclusion
The AspLFD demonstrated good performance in diagnosing IA in cancer patients, especially those with solid tumours. The AspLFD is thus an alternative POCT, particularly when GM evaluations are not readily available.

Similar content being viewed by others
Data availability
The data that support the findings of this study are available upon reasonable request from the corresponding author.
References
Dagenais TR, Keller NP (2009) Pathogenesis of aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev 22(3):447–465. https://doi.org/10.1128/cmr.00055-08
Douglas AP, Smibert OC, Bajel A et al (2021) Consensus guidelines for the diagnosis and management of invasive aspergillosis. Intern Med J 51(Suppl 7):143–176. https://doi.org/10.1111/imj.15591
Liu B, Totten M, Nematollahi S et al (2020) Development and evaluation of a fully automated molecular assay targeting the mitochondrial small subunit rRNA gene for the detection of Pneumocystis Jirovecii in bronchoalveolar lavage fluid specimens. J Mol Diagn 22(12):1482–1493. https://doi.org/10.1016/j.jmoldx.2020.10.003[Erratum in J Mol Diagn 2021
Xue T, Kong X, Ma L (2023) Trends in the epidemiology of Pneumocystis pneumonia in immunocompromised patients without HIV infection. J Fungi (Basel) 9(8):812. https://doi.org/10.3390/jof9080812
Chen CA, Ho CH, Wu YC, Chen YC, Wang JJ, Liao KM (2022) Epidemiology of aspergillosis in cancer patients in Taiwan. Infect Drug Resist 15:3757–3766. https://doi.org/10.2147/IDR.S370967
Arvanitis M, Mylonakis E (2015) Diagnosis of invasive aspergillosis: recent developments and ongoing challenges. Eur J Clin Invest 45(6):646–652. https://doi.org/10.1111/eci.12448
Inoue K, Muramatsu K, Nishimura T et al (2022) Association between early diagnosis of and inpatient mortality from invasive pulmonary aspergillosis among patients without immunocompromised host factors: a nationwide observational study. Int J Infect Dis 122:279–284. https://doi.org/10.1016/j.ijid.2022.05.048
Calero AL, Alonso R, Gadea I et al (2022) Comparison of the performance of two galactomannan detection tests: Platelia Aspergillus Ag and Aspergillus Galactomannan Ag Virclia Monotest. Microbiol Spectr 10(2):e0262621. https://doi.org/10.1128/spectrum.02626-21
Kanaujia R, Singh S, Rudramurthy SM (2023) Aspergillosis: an update on clinical spectrum, diagnostic schemes, and management. Curr Fungal Infect Rep 4:1–12. https://doi.org/10.1007/s12281-023-00461-5
Horvath JA, Dummer S (1996) The use of respiratory-tract cultures in the diagnosis of invasive pulmonary aspergillosis. Am J Med 100(2):171–178. https://doi.org/10.1016/S0002-9343(97)89455-7
Perfect JR, Cox GM, Lee JY et al (2001) The impact of culture isolation of aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis 33(11):1824–1833. https://doi.org/10.1086/323900
Donnelly JP, Chen SC, Kauffman CA et al (2020) Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 71(6):1367–1376. https://doi.org/10.1093/cid/ciz1008
Stynen D, Sarfati J, Goris A et al (1992) Rat monoclonal antibodies against aspergillus galactomannan. Infect Immun 60(6):2237–2245. https://doi.org/10.1128/iai.60.6.2237-2245.1992
Ku NS, Han SH, Choi JY et al (2012) Diagnostic value of the serum galactomannan assay for invasive aspergillosis: it is less useful in non-haematological patients. Scand J Infect Dis 44(8):600–604. https://doi.org/10.3109/00365548.2012.657672
Egger M, Penziner S, Dichtl K et al (2022) Performance of the Euroimmun aspergillus antigen ELISA for the diagnosis of invasive pulmonary aspergillosis in bronchoalveolar lavage fluid. J Clin Microbiol 60(4):e0021522. https://doi.org/10.1128/jcm.00215-22
Buil JB, Huygens S, Dunbar A et al (2023) Retrospective multicenter evaluation of the VirClia galactomannan antigen assay for the diagnosis of pulmonary aspergillosis with bronchoalveolar lavage fluid samples from patients with hematological disease. J Clin Microbiol 61(5):e0004423. https://doi.org/10.1128/jcm.00044-23
Thornton CR (2008) Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin Vaccine Immunol 15(7):1095–1105. https://doi.org/10.1128/CVI.00068-08
Roiz-Mesones MP, Pintos-Fonseca AM, Ahedo-García N, Alegría-Puig CR (2023) Evaluation of the EUROIMMUN aspergillus antigen immunoenzyme assay in serum and bronchoalveolar lavage fluid samples. Enferm Infecc Microbiol Clin (Engl Ed) 41(3):176–180. https://doi.org/10.1016/j.eimce.2021.08.018
White PL, Parr C, Thornton C, Barnes RA (2013) Evaluation of real-time PCR, galactomannan enzyme-linked immunosorbent assay (ELISA), and a novel lateral-flow device for diagnosis of invasive aspergillosis. J Clin Microbiol 51(5):1510–1516. https://doi.org/10.1128/jcm.03189-12
Willinger B, Lackner M, Lass-Flörl C et al (2014) Bronchoalveolar lavage lateral-flow device test for invasive pulmonary aspergillosis in solid organ transplant patients: a semiprospective multicenter study. Transplantation 98(8):898–902. https://doi.org/10.1097/TP.0000000000000153
Prattes J, Flick H, Prüller F et al (2014) Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am J Respir Crit Care Med 190(8):922–929. https://doi.org/10.1164/rccm.201407-1275OC
Eigl S, Prattes J, Lackner M et al (2015) Multicenter evaluation of a lateral-flow device test for diagnosing invasive pulmonary aspergillosis in ICU patients. Crit Care 19(1):178. https://doi.org/10.1186/s13054-015-0905-x
Mercier T, Schauwvlieghe A, de Kort E et al (2019) Diagnosing invasive pulmonary aspergillosis in hematology patients: a retrospective multicenter evaluation of a novel lateral flow device. J Clin Microbiol 57(4):e01913–e01918. https://doi.org/10.1128/jcm.01913-18
Aerts R, Mercier T, Houben E, Schauwvlieghe A, Lagrou K, Maertens J (2022) Performance of the JF5-based galactomannoprotein EIA compared to the lateral flow device and the galactomannan EIA in serum and bronchoalveolar lavage fluid. J Clin Microbiol 60(11):e0094822. https://doi.org/10.1128/jcm.00948-22
Hoenigl M, Koidl C, Duettmann W et al (2012) Bronchoalveolar lavage lateral-flow device test for invasive pulmonary aspergillosis diagnosis in haematological malignancy and solid organ transplant patients. J Infect 65(6):588–591. https://doi.org/10.1016/j.jinf.2012.10.003
Hoenigl M, Prattes J, Spiess B et al (2014) Performance of galactomannan, beta-D-glucan, aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol 52(6):2039–2045. https://doi.org/10.1128/jcm.00467-14
Held J, Schmidt T, Thornton CR, Kotter E, Bertz H (2013) Comparison of a novel aspergillus lateral-flow device and the Platelia® Galactomannan assay for the diagnosis of invasive aspergillosis following haematopoietic stem cell transplantation. Infection 41(6):1163–1169. https://doi.org/10.1007/s15010-013-0472-5
Pan Z, Fu M, Zhang J, Zhou H, Fu Y, Zhou J (2015) Diagnostic accuracy of a novel lateral-flow device in invasive aspergillosis: a meta-analysis. J Med Microbiol 64(7):702–707. https://doi.org/10.1099/jmm.0.000092
Heldt S, Hoenigl M (2017) Lateral Flow assays for the diagnosis of invasive aspergillosis: current status. Curr Fungal Infect Rep 11(2):45–51. https://doi.org/10.1007/s12281-017-0275-8
Marr KA, Laverdiere M, Gugel A, Leisenring W (2005) Antifungal therapy decreases sensitivity of the aspergillus galactomannan enzyme immunoassay. Clin Infect Dis 40(12):1762–1769. https://doi.org/10.1086/429921
Acet-Öztürk NA, Ömer-Topçu D, Vurat Acar K et al (2024) Impact of posaconazole prophylaxis and antifungal treatment on BAL GM performance in hematology malignancy patients with febrile neutropenia: a real life experience. Eur J Clin Microbiol Infect Dis 43(1):33–43. https://doi.org/10.1007/s10096-023-04686-7
Acknowledgements
The authors thank the Richardson Guangzhou Centre for Fungal Diagnostics and Research for providing the AspLFD kit for research. This centre had no role in the study design, data collection and analysis, publish decision, or manuscript preparation.
Funding
This study was supported in part by the Natural Science Foundation of Anhui Province (grant number 2208085MH253), People’s Republic of China.
Author information
Authors and Affiliations
Contributions
Lijuan Wan: Conceptualization, Data Curation, Formal Analysis, Writing–Original Draft Preparation; Xueqin Cai: Data Curation, Methodology; Meng Ling: Formal Analysis, Writing–Review & Editing; Jinsong Kan: Validation; Meiling Yin: Visualization; Huiyan Wang: Conceptualization, Investigation, Writing–Review & Editing, Funding Acquisition.
Corresponding author
Ethics declarations
Ethical approval
This study involving human participants was reviewed and approved by Institutional Ethics Committee (IEC) of the First Affiliated Hospital of USTC (code 2024-013). This study was conducted in line with the Declaration of Helsinki.
Consent to participate
Informed consent was waived by the committee because of the retrospective use of remnant and de-identified samples. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wan, L., Cai, X., Ling, M. et al. Evaluation of the JF5-based Aspergillus galactomannoprotein lateral flow device for diagnosing invasive aspergillosis in cancer patients. Eur J Clin Microbiol Infect Dis (2024). https://doi.org/10.1007/s10096-024-04830-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10096-024-04830-x