Abstract
Objective
Previous studies have demonstrated an association between sex hormone-related traits and systemic lupus erythematosus (SLE). However, because of the difficulties in determining sequential temporality, the causal association remains elusive. In this study, we used two-sample Mendelian randomization (MR) to explore the genetic causal associations between sex hormone-related traits and SLE.
Methods
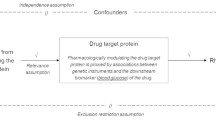
We used a two-sample MR to explore the causal association between sex hormone-related traits and SLE. The summarized data for sex hormone-related traits (including testosterone, estradiol (E2), sex hormone-binding globulin (SHBG), and bioavailable testosterone (BT)) originated from large genome-wide association studies (GWASs) of European descent. Aggregated data for SLE were derived from the FinnGen consortium (835 cases and 300,162 controls). Random-effects inverse-variance weighted (IVW), MR-Egger, weighted median, simple mode, weighted mode, and fixed-effects IVW methods were used for the MR analysis. Random-effects IVW was the primary method used to analyze the genetic causal association between sex hormone-related traits and SLE. Heterogeneity of the MR results was detected using the IVW Cochran’s Q estimates. The pleiotropy of MR results was detected using MR-Egger regression and the MR pleiotropy residual sum and outlier (MR-PRESSO) test. Finally, leave-one-out analysis was performed to determine whether MR results were affected by a single single-nucleotide polymorphism (SNP).
Results
Random-effects IVW as the primary method showed that testosterone (odds ratio (OR), 0.87; 95% confidence interval (CI), 0.41–1.82; P = 0.705), E2 (OR, 0.95; 95% CI, 0.73–1.23; P = 0.693), SHBG (OR, 1.25; 95% CI, 0.74–2.13; P = 0.400), and BT (OR, 0.99; 95% CI, 0.67–1.47; P = 0.959) had no potential causal association with SLE. The MR-Egger, weighted median, simple mode, weighted mode, and fixed-effects IVW methods all indicated consistent results. The results of the MR-Egger regression showed that there was no pleiotropy in our MR analysis (P > 0.05). The IVW Cochran’s Q estimates showed that the MR analysis results of E2, SHBG, and BT on SLE had no heterogeneity (P > 0.05), but testosterone and SLE had heterogeneity (P < 0.05). The leave-one-out analysis confirmed that a single SNP did not affect the MR results.
Conclusions
Our MR analysis demonstrated that genetically predicted testosterone, E2, SHBG, and BT levels were not associated with SLE risk, but the roles of other non-genetic pathways cannot be ruled out.
Key Points • This is the first MR study to explore the causal association of sex hormone-related traits with SLE. • No evidence to support causal associations between sex hormone-related traits and SLE. • Our MR analysis may provide novel insights into the causal association between sex hormone-related traits and SLE risk. |





Similar content being viewed by others
Data availability
The datasets supporting this study are available from IEU OpenGWAS (https://gwas.mrcieu.ac.uk/) and FinnGen consortium (https:// www.finngen.fi/).
References
Tsokos GC (2011) Systemic lupus erythematosus. N Engl J Med 365:2110–2121. https://doi.org/10.1056/NEJMra1100359
Durcan L, O’Dwyer T, Petri M (2019) Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 393:2332–2343. https://doi.org/10.1016/S0140-6736(19)30237-5
Kiriakidou M, Ching CL (2020) Systemic lupus erythematosus. Ann Intern Med 172:ITC81-ITC96. https://doi.org/10.7326/AITC202006020
Wang P, Dan Y-L, Wu Q, Tao S-S, Yang X-K, Wang D-G, Ye D-Q, Shuai Z-W, Pan H-F (2021) Non-causal effects of smoking and alcohol use on the risk of systemic lupus erythematosus. Autoimmun Rev 20:102890. https://doi.org/10.1016/j.autrev.2021.102890
Tsokos GC (2020) Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol 21:605–614. https://doi.org/10.1038/s41590-020-0677-6
Kwon Y-C, Chun S, Kim K, Mak A (2019) Update on the genetics of systemic lupus erythematosus: genome-wide association studies and beyond. Cells 8. https://doi.org/10.3390/cells8101180
Bakshi J, Segura BT, Wincup C, Rahman A (2018) Unmet needs in the pathogenesis and treatment of systemic lupus erythematosus. Clin Rev Allergy Immunol 55:352–367. https://doi.org/10.1007/s12016-017-8640-5
Gubbels Bupp MR, Jorgensen TN (2018) Androgen-induced immunosuppression. Front Immunol 9:794. https://doi.org/10.3389/fimmu.2018.00794
Jones JM, Jørgensen TN (2020) Androgen-mediated anti-inflammatory cellular processes as therapeutic targets in lupus. Front Immunol 11:1271. https://doi.org/10.3389/fimmu.2020.01271
Maselli A, Conti F, Alessandri C, Colasanti T, Barbati C, Vomero M, Ciarlo L, Patrizio M, Spinelli FR, Ortona E et al (2016) Low expression of estrogen receptor β in T lymphocytes and high serum levels of anti-estrogen receptor α antibodies impact disease activity in female patients with systemic lupus erythematosus. Biol Sex Differ 7:3. https://doi.org/10.1186/s13293-016-0057-y
Singh RP, Hahn BH, Bischoff DS (2021) Interferon genes are influenced by 17β-estradiol in SLE. Front Immunol 12:725325. https://doi.org/10.3389/fimmu.2021.725325
Simons PIHG, Valkenburg O, Stehouwer CDA, Brouwers MCGJ (2021) Sex hormone-binding globulin: biomarker and hepatokine? Trends Endocrinol Metab 32:544–553. https://doi.org/10.1016/j.tem.2021.05.002
Piotrowski P, Gasik R, Lianeri M, Cieślak D, Wudarski M, Hrycaj P, Łacki JK, Jagodziński PP (2010) Asp327Asn polymorphism of sex hormone-binding globulin gene is associated with systemic lupus erythematosus incidence. Mol Biol Rep 37:235–239. https://doi.org/10.1007/s11033-009-9639-7
Doukas C, Saltiki K, Mantzou A, Cimponeriu A, Terzidis K, Sarika L, Mavrikakis M, Sfikakis P, Alevizaki M (2013) Hormonal parameters and sex hormone receptor gene polymorphisms in men with autoimmune diseases. Rheumatol Int 33:575–582. https://doi.org/10.1007/s00296-012-2386-4
Gao N, Kong M, Li X, Wei D, Zhu X, Hong Z, Ni M, Wang Y, Dong A (2022) Systemic lupus erythematosus and cardiovascular disease: a Mendelian randomization study. Front Immunol 13:908831. https://doi.org/10.3389/fimmu.2022.908831.
Sekula P, Del Greco MF, Pattaro C, Köttgen A (2016) Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 27:3253–3265
Xiang K, Wang P, Xu Z, Hu Y-Q, He Y-S, Chen Y, Feng Y-T, Yin K-J, Huang J-X, Wang J et al (2021) Causal effects of gut microbiome on systemic lupus erythematosus: a two-sample Mendelian randomization study. Front Immunol 12:667097. https://doi.org/10.3389/fimmu.2021.667097
Dan Y-L, Wang P, Cheng Z, Wu Q, Wang X-R, Wang D-G, Pan H-F (2021) Circulating adiponectin levels and systemic lupus erythematosus: a two-sample Mendelian randomization study. Rheumatology (Oxford) 60:940–946. https://doi.org/10.1093/rheumatology/keaa506
Zhang Y, Fang Y, Xu N, Tian L, Min X, Chen G, Dai T, Liu N, Wang X, Rao Y et al (2023) The causal effects of age at menarche, age at first live birth, and estradiol levels on systemic lupus erythematosus: a two-sample Mendelian randomization analysis. Lupus 9612033231180358. https://doi.org/10.1177/09612033231180358.
Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, Beaumont RN, Wittemans L, Martin S, Busch AS et al (2020) Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med 26:252–258. https://doi.org/10.1038/s41591-020-0751-5
Schmitz D, Ek WE, Berggren E, Höglund J, Karlsson T, Johansson Å (2021) Genome-wide association study of estradiol levels and the causal effect of estradiol on bone mineral density. J Clin Endocrinol Metab 106:e4471–e4486. https://doi.org/10.1210/clinem/dgab507
Woo JMP, Parks CG, Jacobsen S, Costenbader KH, Bernatsky S (2022) The role of environmental exposures and gene-environment interactions in the etiology of systemic lupus erythematous. J Intern Med 291:755–778. https://doi.org/10.1111/joim.13448
Chen J, Liao S, Pang W, Guo F, Yang L, Liu H-F, Pan Q (2022) Life factors acting on systemic lupus erythematosus. Front Immunol 13:986239. https://doi.org/10.3389/fimmu.2022.986239
Leffers HCB, Lange T, Collins C, Ulff-Møller CJ, Jacobsen S (2019) The study of interactions between genome and exposome in the development of systemic lupus erythematosus. Autoimmun Rev 18:382–392. https://doi.org/10.1016/j.autrev.2018.11.005
Rosoff D B, Davey Smith G, Mehta N, Clarke T-K, Lohoff F W (2020) Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: a multivariable Mendelian randomization study. PLoS Med 17:e1003410. https://doi.org/10.1371/journal.pmed.1003410
Liu B, Ye D, Yang H, Song J, Sun X, Mao Y, He Z (2022) Two-sample mendelian randomization analysis investigates causal associations between gut microbial genera and inflammatory bowel disease, and specificity causal associations in ulcerative colitis or Crohn’s disease. Front Immunol 13:921546. https://doi.org/10.3389/fimmu.2022.921546
Chen L, Sun X, Wang Z, Lu Y, Chen M, He Y, Xu H, Zheng L (2021) The impact of plasma vitamin C levels on the risk of cardiovascular diseases and Alzheimer’s disease: a Mendelian randomization study. Clin Nutr (Edinburgh, Scotland) 40:5327–5334. https://doi.org/10.1016/j.clnu.2021.08.020
Chen H, Mi S, Zhu J, Jin W, Li Y, Wang T, Li Y, Fan C (2021) No causal association between adiponectin and the risk of rheumatoid arthritis: a Mendelian randomization study. Front Genet 12:670282. https://doi.org/10.3389/fgene.2021.670282
Yin K-J, Huang J-X, Wang P, Yang X-K, Tao S-S, Li H-M, Ni J, Pan H-F (2022) No genetic causal association between periodontitis and arthritis: a bidirectional two-sample Mendelian randomization analysis. Front Immunol 13:808832. https://doi.org/10.3389/fimmu.2022.808832
Cui Z, Hou G, Meng X, Feng H, He B, Tian Y (2020) Bidirectional causal associations between inflammatory bowel disease and ankylosing spondylitis: a two-sample Mendelian randomization analysis. Front Genet 11:587876. https://doi.org/10.3389/fgene.2020.587876
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR (2016) Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol 45:1961–1974. https://doi.org/10.1093/ije/dyw220
Li C, Niu M, Guo Z, Liu P, Zheng Y, Liu D, Yang S, Wang W, Li Y, Hou H (2022) A mild causal relationship between tea consumption and obesity in general population: a two-sample Mendelian randomization study. Front Genet 13:795049. https://doi.org/10.3389/fgene.2022.795049
Hartwig FP, Davey Smith G, Bowden J (2017) Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol 46:1985–1998. https://doi.org/10.1093/ije/dyx102
Gao R-C, Sang N, Jia C-Z, Zhang M-Y, Li B-H, Wei M, Wu G-C (2022) Association between sleep traits and rheumatoid arthritis: a Mendelian randomization study. Front Public Health 10: 940161. https://doi.org/10.3389/fpubh.2022.940161
Shu M-J, Li J-R, Zhu Y-C, Shen H (2022) Migraine and ischemic stroke: a Mendelian randomization study. Neurol Ther 11:237–246. https://doi.org/10.1007/s40120-021-00310-y
Chen L, Yang H, Li H, He C, Yang L, Lv G (2022) Insights into modifiable risk factors of cholelithiasis: a Mendelian randomization study. Hepatology (Baltimore, Md.) 75:785–796. https://doi.org/10.1002/hep.32183
Hong J, Qu Z, Ji X, Li C, Zhang G, Jin C, Wang J, Zhang Y, Shen Y, Meng J et al (2021) Genetic associations between IL-6 and the development of autoimmune arthritis are gender-specific. Front Immunol 12:707617. https://doi.org/10.3389/fimmu.2021.707617
Dong Z, Zhang B, Rong J, Yang X, Wang Y, Zhang Q, Su Z (2022) The aberrant expression of CD45 isoforms and levels of sex hormones in systemic lupus erythematosus. Clin Rheumatol 41:1087–1093. https://doi.org/10.1007/s10067-021-05934-x
Pakpoor J, Goldacre R, Goldacre MJ (2018) Associations between clinically diagnosed testicular hypofunction and systemic lupus erythematosus: a record linkage study. Clin Rheumatol 37:559–562. https://doi.org/10.1007/s10067-017-3873-5
Singh RP, Bischoff D S (2021) Sex hormones and gender influence the expression of markers of regulatory T cells in SLE patients. Front Immunol 12:619268. https://doi.org/10.3389/fimmu.2021.619268
Merrill JT, Lahita RG (2000) Sex hormone binding globulins and atherosclerotic risk in systemic lupus. Lupus 9:217–222
Arnaud L, Nordin A, Lundholm H, Svenungsson E, Hellbacher E, Wikner J, Zickert A, Gunnarsson I (2017) Effect of corticosteroids and cyclophosphamide on sex hormone profiles in male patients with systemic lupus erythematosus or systemic sclerosis. Arthritis Rheumatol 69:1272–1279. https://doi.org/10.1002/art.40057
Gordon C, Wallace DJ, Shinada S, Kalunian KC, Forbess L, Braunstein GD, Weisman MH (2008) Testosterone patches in the management of patients with mild/moderate systemic lupus erythematosus. Rheumatology (Oxford) 47:334–338. https://doi.org/10.1093/rheumatology/kem342
Ding Y, He J, Guo J-P, Dai Y-J, Li C, Feng M, Li R, Li Z-G (2012) Gender differences are associated with the clinical features of systemic lupus erythematosus. Chin Med J 125:2477–2481
Jones JM, Smith F, Littlejohn E, Jorgensen TN (2022) Lack of association between sex hormones, MDSCs, LDGs and pDCs in males and females with systemic lupus erythematosus. Front Immunol 13:888501. https://doi.org/10.3389/fimmu.2022.888501
Robeva R, Tanev D, Andonova S, Kirilov G, Savov A, Stoycheva M, Tomova A, Kumanov P, Rashkov R, Kolarov Z (2013) Androgen receptor (CAG)n polymorphism and androgen levels in women with systemic lupus erythematosus and healthy controls. Rheumatol Int 33:2031–2038. https://doi.org/10.1007/s00296-013-2687-2
Moulton VR (2018) Sex hormones in acquired immunity and autoimmune disease. Front Immunol 9:2279. https://doi.org/10.3389/fimmu.2018.02279
Kim J-W, Kim H-A, Suh C-H, Jung J-Y (2022) Sex hormones affect the pathogenesis and clinical characteristics of systemic lupus erythematosus. Front Med 9:906475. https://doi.org/10.3389/fmed.2022.906475
Manuel RSJ, Liang Y (2021) Sexual dimorphism in immunometabolism and autoimmunity: Impact on personalized medicine. Autoimmun Rev 20:102775. https://doi.org/10.1016/j.autrev.2021.102775
Talaat RM, Mohamed SF, Bassyouni IH, Raouf AA (2015) Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: correlation with disease activity. Cytokine 72:146–153. https://doi.org/10.1016/j.cyto.2014.12.027
Cutolo M, Straub RH (2020) Sex steroids and autoimmune rheumatic diseases: state of the art. Nat Rev Rheumatol 16:628–644. https://doi.org/10.1038/s41584-020-0503-4
Ripley BJM, Goncalves B, Isenberg DA, Latchman DS, Rahman A (2005) Raised levels of interleukin 6 in systemic lupus erythematosus correlate with anaemia. Ann Rheum Dis 64:849–853
Mao X, Wu Y, Diao H, Hao J, Tian G, Jia Z, Li Z, Xiong S, Wu Z, Wang P et al (2014) Interleukin-6 promotes systemic lupus erythematosus progression with Treg suppression approach in a murine systemic lupus erythematosus model. Clin Rheumatol 33:1585–1593. https://doi.org/10.1007/s10067-014-2717-9
Mok CC, Lau CS (2000) Profile of sex hormones in male patients with systemic lupus erythematosus. Lupus 9:252–257
Bricou O, Taïeb O, Baubet T, Gal B, Guillevin L, Moro MR (2006) Stress and coping strategies in systemic lupus erythematosus: a review. NeuroImmunoModulation 13:283–293
Molina E, Gould N, Lee K, Krimins R, Hardenbergh D, Timlin H (2022) Stress, mindfulness, and systemic lupus erythematosus: an overview and directions for future research. Lupus 31:1549–1562. https://doi.org/10.1177/09612033221122980
Patterson S, Trupin L, Hartogensis W, DeQuattro K, Lanata C, Gordon C, Barbour KE, Greenlund KJ, Dall’Era M, Yazdany J et al (2022) Perceived stress and prediction of worse disease activity and symptoms in a multiracial, multiethnic systemic lupus erythematosus cohort. Arthritis Care Res (Hoboken). https://doi.org/10.1002/acr.25076
Roberts AL, Malspeis S, Kubzansky LD, Feldman CH, Chang S-C, Koenen KC, Costenbader KH (2017) Association of trauma and posttraumatic stress disorder with incident systemic lupus erythematosus in a longitudinal cohort of women. Arthritis Rheumatol 69:2162–2169. https://doi.org/10.1002/art.40222
Ilchmann-Diounou H, Menard S (2020) Psychological stress, intestinal barrier dysfunctions, and autoimmune disorders: an overview. Front Immunol 11:1823. https://doi.org/10.3389/fimmu.2020.01823
Parks CG, de Souza Espindola Santos A, Barbhaiya M, Costenbader KH (2017) Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 31:306-320. https://doi.org/10.1016/j.berh.2017.09.005
Rigante D, Esposito S (2015) Infections and systemic lupus erythematosus: binding or sparring partners? Int J Mol Sci 16:17331–17343. https://doi.org/10.3390/ijms160817331
Draborg AH, Sandhu N, Larsen N, Lisander Larsen J, Jacobsen S, Houen G (2016) Impaired cytokine responses to Epstein-Barr virus antigens in systemic lupus erythematosus patients. J Immunol Res 2016:6473204. https://doi.org/10.1155/2016/6473204
Cui J, Yan W, Xu S, Wang Q, Zhang W, Liu W, Ni A (2018) Anti-Epstein-Barr virus antibodies in Beijing during 2013–2017: what we have found in the different patients. PLoS One 13:e0193171. https://doi.org/10.1371/journal.pone.0193171
Illescas-Montes R, Corona-Castro CC, Melguizo-Rodríguez L, Ruiz C, Costela-Ruiz VJ (2019) Infectious processes and systemic lupus erythematosus. Immunology 158:153–160. https://doi.org/10.1111/imm.13103
Nelson P, Rylance P, Roden D, Trela M, Tugnet N (2014) Viruses as potential pathogenic agents in systemic lupus erythematosus. Lupus 23:596–605. https://doi.org/10.1177/0961203314531637
Saha S, Tieng A, Pepeljugoski K P, Zandamn-Goddard G, Peeva E (2011) Prolactin, systemic lupus erythematosus, and autoreactive B cells: lessons learnt from murine models. Clin Rev Allergy Immunol 40. https://doi.org/10.1007/s12016-009-8182-6
McMurray RW, Keisler D, Izui S, Walker SE (1993) Effects of parturition, suckling and pseudopregnancy on variables of disease activity in the B/W mouse model of systemic lupus erythematosus. J Rheumatol 20:1143–1151
McMurray R, Keisler D, Kanuckel K, Izui S, Walker SE (1991) Prolactin influences autoimmune disease activity in the female B/W mouse. J Immunol 147:3780–3787
Wang P, Lv T-T, Guan S-Y, Li H-M, Leng R-X, Zou Y-F, Pan H-F (2017) Increased plasma/serum levels of prolactin in systemic lupus erythematosus: a systematic review and meta-analysis. Postgrad Med 129:126–132. https://doi.org/10.1080/00325481.2017.1241130
Song GG, Lee YH (2017) Circulating prolactin level in systemic lupus erythematosus and its correlation with disease activity: a meta-analysis. Lupus 26:1260–1268. https://doi.org/10.1177/0961203317693094
Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G (2008) Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27:1133–1163
Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37:658–665. https://doi.org/10.1002/gepi.21758
Author information
Authors and Affiliations
Contributions
Conceptualization: Guolian Yuan. Methodology: Guolian Yuan, Mingyi Yang, and Jiale Xie. Formal analysis and investigation: Guolian Yuan, Mingyi Yang, and Jiale Xie. Writing—original draft preparation: Guolian Yuan. Writing—review and editing: Guolian Yuan. Resources: Ke Xu and Feng Zhang. Supervision: Ke Xu and Feng Zhang.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, G., Yang, M., Xie, J. et al. No evidence of genetic causal association between sex hormone-related traits and systemic lupus erythematosus: A two-sample Mendelian randomization study. Clin Rheumatol 42, 3237–3249 (2023). https://doi.org/10.1007/s10067-023-06700-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06700-x