Abstract
Objective
To describe the clinical characteristics and risk factors of clinical recurrence in interstitial lung disease related to antisynthetase syndrome (ARS-ILD).
Methods
Patients diagnosed as ARS-ILD in Nanjing Drum Tower Hospital between January 2015 and November 2020 were retrospectively analyzed. Clinical information and treatment course were reviewed. The primary endpoint was the disease recurrence, and the secondary point was mortality. Univariate and multivariable Cox regression analyses were performed to identify risk factors for recurrence.
Results
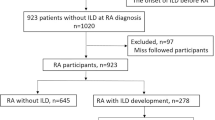
Totally, 132 patients with ARS-ILD received immunomodulation treatment from diagnosis. During follow-ups, sixty-nine patients showed recurrence, with a recurrency rate yielding 52.3%. The median duration from treatment initiation to recurrence was 11 (5–18) months. The median tapering course in the recurrence group was 8 (3–12.5) months, which was significantly shorter than the 16 (10–32) months in the no-recurrence group (p < 0.001). Fifty-eight patients experienced recurrence when the glucocorticoids (GC) dose dropped to 10 (9.375–15) mg/day. Twelve patients discontinued GC with a median treatment course of 11.5 (8–16.75) months, and 11 patients developed recurrence after discontinuing GC for 3 (1–4) months. Twelve patients died, with a mortality rate of 9.1%, and recurrence was not associated with increased mortality. The adjusted multivariate analysis showed that age, increased serum lactate dehydrogenase (LDH) level, relatively shorter tapering duration, and inappropriate GC discontinuation were associated with recurrence.
Conclusion
Recurrence of ARS-ILD was common during medication intensity reduction. Age, LDH, medication tapering duration, and discontinuation were risk factors for recurrence. Further efforts to reduce recurrence should take into consideration of these factors.
Key Points • Recurrence is observed commonly with a recurrency rate 52.3% in patients with interstitial lung disease related to antisynthetase syndrome (ARS-ILD) when glucocorticoids (GC) tapering or discontinuation. • Age, increased serum lactate dehydrogenase (LDH) level, medication tapering duration, and GC discontinuation were identified to be significantly associated with the recurrence of ARS-ILD. |


Similar content being viewed by others
References
Mariampillai K, Granger B, Amelin D, Guiguet M, Hachulla E, Maurier F, Meyer A, Tohme A, Charuel JL, Musset L, Allenbach Y, Benveniste O (2018) Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis-specific autoantibodies. JAMA Neurol 75(12):1528–1537. https://doi.org/10.1001/jamaneurol.2018.2598
Lundberg IE, Tjarnlund A, Bottai M, Werth VP, Pilkington C, de Visser M, Alfredsson L, Amato AA, Barohn RJ, Liang MH, Singh JA, Aggarwal R, Arnardottir S, Chinoy H, Cooper RG, Danko K, Dimachkie MM, Feldman BM, Garcia-De La Torre I, Gordon P, Hayashi T, Katz JD, Kohsaka H, Lachenbruch PA, Lang BA, Li Y, Oddis CV, Olesinska M, Reed AM, Rutkowska-Sak L, Sanner H, Selva-O’Callaghan A, Song YW, Vencovsky J, Ytterberg SR, Miller FW, Rider LG, International Myositis Classification Criteria Project Consortium tER, the Juvenile Dermatomyositis Cohort Biomarker S, Repository (2017) 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol 69(12):2271–2282. https://doi.org/10.1002/art.40320
Marguerie C, Bunn CC, Beynon HL, Bernstein RM, Hughes JM, So AK, Walport MJ (1990) Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med 77(282):1019–1038. https://doi.org/10.1093/qjmed/77.1.1019
Mejia M, Herrera-Bringas D, Perez-Roman DI, Rivero H, Mateos-Toledo H, Castorena-Garcia P, Figueroa JE, Rojas-Serrano J (2017) Interstitial lung disease and myositis-specific and associated autoantibodies: clinical manifestations, survival and the performance of the new ATS/ERS criteria for interstitial pneumonia with autoimmune features (IPAF). Respir Med 123:79–86. https://doi.org/10.1016/j.rmed.2016.12.014
Ghirardello A, Doria A (2018) New insights in myositis-specific autoantibodies. Curr Opin Rheumatol 30(6):614–622. https://doi.org/10.1097/BOR.0000000000000548
Shi J, Li S, Yang H, Zhang Y, Peng Q, Lu X, Wang G (2017) Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies. J Rheumatol 44(7):1051–1057. https://doi.org/10.3899/jrheum.161480
Pipitone N, Salvarani C (2020) Up-to-date treatment and management of myositis. Curr Opin Rheumatol 32(6):523–527. https://doi.org/10.1097/BOR.0000000000000745
Zeng R, Glaubitz S, Schmidt J (2021) Inflammatory myopathies: shedding light on promising agents and combination therapies in clinical trials. Expert Opin Investig Drugs 30(11):1125–1140. https://doi.org/10.1080/13543784.2021.2003776
Liu Y, Liu X, Xie M, Chen Z, He J, Wang Z, Dai J, Cai H (2020) Clinical characteristics of patients with anti-EJ antisynthetase syndrome associated interstitial lung disease and literature review. Respir Med 165:105920. https://doi.org/10.1016/j.rmed.2020.105920
Yoshifuji H, Fujii T, Kobayashi S, Imura Y, Fujita Y, Kawabata D, Usui T, Tanaka M, Nagai S, Umehara H, Mimori T (2006) Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 39(3):233–241. https://doi.org/10.1080/08916930600622884
Nakazawa M, Kaneko Y, Takeuchi T (2018) Risk factors for the recurrence of interstitial lung disease in patients with polymyositis and dermatomyositis: a retrospective cohort study. Clin Rheumatol 37(3):765–771. https://doi.org/10.1007/s10067-017-3854-8
Koreeda Y, Higashimoto I, Yamamoto M, Takahashi M, Kaji K, Fujimoto M, Kuwana M, Fukuda Y (2010) Clinical and pathological findings of interstitial lung disease patients with anti-aminoacyl-tRNA synthetase autoantibodies. Intern Med 49(5):361–369. https://doi.org/10.2169/internalmedicine.49.2889
Sasano H, Hagiwara E, Kitamura H, Enomoto Y, Matsuo N, Baba T, Iso S, Okudela K, Iwasawa T, Sato S, Suzuki Y, Takemura T, Ogura T (2016) Long-term clinical course of anti-glycyl tRNA synthetase (anti-EJ) antibody-related interstitial lung disease pathologically proven by surgical lung biopsy. BMC Pulm Med 16(1):168. https://doi.org/10.1186/s12890-016-0325-y
Hozumi H, Fujisawa T, Nakashima R, Yasui H, Suzuki Y, Kono M, Karayama M, Furuhashi K, Enomoto N, Inui N, Nakamura Y, Mimori T, Suda T (2019) Efficacy of glucocorticoids and calcineurin inhibitors for anti-aminoacyl-tRNA synthetase antibody-positive polymyositis/dermatomyositis-associated interstitial lung disease: a propensity score-matched analysis. J Rheumatol 46(5):509–517. https://doi.org/10.3899/jrheum.180778
Takei R, Yamano Y, Kataoka K, Yokoyama T, Matsuda T, Kimura T, Johkoh T, Takahashi O, Kondoh Y (2020) Predictive factors for the recurrence of anti-aminoacyl-tRNA synthetase antibody-associated interstitial lung disease. Respir Investig 58(2):83–90. https://doi.org/10.1016/j.resinv.2019.10.004
Sun S, Chen Z, Zhang D, Xu W, Wu W, Sun F, Gu L, Chen J, Li J, Li T, Wang X, Ye S (2021) Description and analysis of a novel subtype of the anti-synthetase syndrome characterized by frequent attacks of fever and systemic inflammation in a single-center cohort study. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.729602
Solomon J, Swigris JJ, Brown KK (2011) Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol 37(1):100–109. https://doi.org/10.1590/s1806-37132011000100015
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (second of two parts). N Engl J Med 292(8):403–407. https://doi.org/10.1056/NEJM197502202920807
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292(7):344–347. https://doi.org/10.1056/NEJM197502132920706
Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D, Pneumonias AECoII (2013) An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188(6):733–748. https://doi.org/10.1164/rccm.201308-1483ST
Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, Kreuter M, Lynch DA, Maher TM, Martinez FJ, Molina-Molina M, Myers JL, Nicholson AG, Ryerson CJ, Strek ME, Troy LK, Wijsenbeek M, Mammen MJ, Hossain T, Bissell BD, Herman DD, Hon SM, Kheir F, Khor YH, Macrea M, Antoniou KM, Bouros D, Buendia-Roldan I, Caro F, Crestani B, Ho L, Morisset J, Olson AL, Podolanczuk A, Poletti V, Selman M, Ewing T, Jones S, Knight SL, Ghazipura M, Wilson KC (2022) Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 205(9):e18–e47. https://doi.org/10.1164/rccm.202202-0399ST
Marco JL, Collins BF (2020) Clinical manifestations and treatment of antisynthetase syndrome. Best Pract Res Clin Rheumatol 34(4):101503. https://doi.org/10.1016/j.berh.2020.101503
Marie I, Josse S, Decaux O, Dominique S, Diot E, Landron C, Roblot P, Jouneau S, Hatron PY, Tiev KP, Vittecoq O, Noel D, Mouthon L, Menard JF, Jouen F (2012) Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev 11(10):739–745. https://doi.org/10.1016/j.autrev.2012.01.006
Zhang Y, Ge Y, Yang H, Chen H, Tian X, Huang Z, Liu S, Lu X, Wang G (2020) Clinical features and outcomes of the patients with anti-glycyl tRNA synthetase syndrome. Clin Rheumatol 39(8):2417–2424. https://doi.org/10.1007/s10067-020-04979-8
Yousaf MN, Powell MD (2012) The effects of heart and skeletal muscle inflammation and cardiomyopathy syndrome on creatine kinase and lactate dehydrogenase levels in Atlantic salmon (Salmo salar L.). Sci World J 2012:741302. https://doi.org/10.1100/2012/741302
Liu H, Xie S, Liang T, Ma L, Sun H, Dai H, Wang C (2019) Prognostic factors of interstitial lung disease progression at sequential HRCT in anti-synthetase syndrome. Eur Radiol 29(10):5349–5357. https://doi.org/10.1007/s00330-019-06152-5
Marie I, Hatron PY, Cherin P, Hachulla E, Diot E, Vittecoq O, Menard JF, Jouen F, Dominique S (2013) Functional outcome and prognostic factors in anti-Jo1 patients with antisynthetase syndrome. Arthritis Res Ther 15(5):R149. https://doi.org/10.1186/ar4332
Spath M, Schroder M, Schlotter-Weigel B, Walter MC, Hautmann H, Leinsinger G, Pongratz D, Muller-Felber W (2004) The long-term outcome of anti-Jo-1-positive inflammatory myopathies. J Neurol 251(7):859–864. https://doi.org/10.1007/s00415-004-0449-5
Oddis CV, Aggarwal R (2018) Treatment in myositis. Nat Rev Rheumatol 14(5):279–289. https://doi.org/10.1038/nrrheum.2018.42
Witt LJ, Curran JJ, Strek ME (2016) The diagnosis and treatment of antisynthetase syndrome. Clin Pulm Med 23(5):218–226. https://doi.org/10.1097/CPM.0000000000000171
Lundberg IE, Tjarnlund A, Bottai M, Werth VP, Pilkington C, Visser M, Alfredsson L, Amato AA, Barohn RJ, Liang MH, Singh JA, Aggarwal R, Arnardottir S, Chinoy H, Cooper RG, Danko K, Dimachkie MM, Feldman BM, Torre IG, Gordon P, Hayashi T, Katz JD, Kohsaka H, Lachenbruch PA, Lang BA, Li Y, Oddis CV, Olesinska M, Reed AM, Rutkowska-Sak L, Sanner H, Selva-O’Callaghan A, Song YW, Vencovsky J, Ytterberg SR, Miller FW, Rider LG, International Myositis Classification Criteria Project consortium TEr, The Juvenile Dermatomyositis Cohort Biomarker S, Repository (2017) 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 76(12):1955–1964. https://doi.org/10.1136/annrheumdis-2017-211468
Kurita T, Yasuda S, Oba K, Odani T, Kono M, Otomo K, Fujieda Y, Oku K, Bohgaki T, Amengual O, Horita T, Atsumi T (2015) The efficacy of tacrolimus in patients with interstitial lung diseases complicated with polymyositis or dermatomyositis. Rheumatology (Oxford) 54(1):39–44. https://doi.org/10.1093/rheumatology/keu166
Cavagna L, Caporali R, Abdi-Ali L, Dore R, Meloni F, Montecucco C (2013) Cyclosporine in anti-Jo1-positive patients with corticosteroid-refractory interstitial lung disease. J Rheumatol 40(4):484–492. https://doi.org/10.3899/jrheum.121026
Doyle TJ, Dhillon N, Madan R, Cabral F, Fletcher EA, Koontz DC, Aggarwal R, Osorio JC, Rosas IO, Oddis CV, Dellaripa PF (2018) Rituximab in the treatment of interstitial lung disease associated with antisynthetase syndrome: a multicenter retrospective case review. J Rheumatol 45(6):841–850. https://doi.org/10.3899/jrheum.170541
Fujisawa T, Hozumi H, Kono M, Enomoto N, Hashimoto D, Nakamura Y, Inui N, Yokomura K, Koshimizu N, Toyoshima M, Shirai T, Yasuda K, Hayakawa H, Suda T (2014) Prognostic factors for myositis-associated interstitial lung disease. PLoS One 9(6):e98824. https://doi.org/10.1371/journal.pone.0098824
Gonzalez-Perez MI, Mejia-Hurtado JG, Perez-Roman DI, Buendia-Roldan I, Mejia M, Falfan-Valencia R, Mateos-Toledo HN, Rojas-Serrano J (2020) Evolution of pulmonary function in a cohort of patients with interstitial lung disease and positive for antisynthetase antibodies. J Rheumatol 47(3):415–423. https://doi.org/10.3899/jrheum.181141
Andersson H, Aalokken TM, Gunther A, Mynarek GK, Garen T, Lund MB, Molberg O (2016) Pulmonary involvement in the antisynthetase syndrome: a comparative cross-sectional study. J Rheumatol 43(6):1107–1113. https://doi.org/10.3899/jrheum.151067
Kurita T, Yasuda S, Amengual O, Atsumi T (2015) The efficacy of calcineurin inhibitors for the treatment of interstitial lung disease associated with polymyositis/dermatomyositis. Lupus 24(1):3–9. https://doi.org/10.1177/0961203314554849
Yousem SA, Gibson K, Kaminski N, Oddis CV, Ascherman DP (2010) The pulmonary histopathologic manifestations of the anti-Jo-1 tRNA synthetase syndrome. Mod Pathol 23(6):874–880. https://doi.org/10.1038/modpathol.2010.65
Marie I, Hatron PY, Dominique S, Cherin P, Mouthon L, Menard JF (2011) Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: a series of 107 patients. Arthritis Rheum 63(11):3439–3447. https://doi.org/10.1002/art.30513
Wilfong EM, Aggarwal R (2021) Role of antifibrotics in the management of idiopathic inflammatory myopathy associated interstitial lung disease. Ther Adv Musculoskelet Dis 13:1759720X211060907. https://doi.org/10.1177/1759720X211060907
Li T, Guo L, Chen Z, Gu L, Sun F, Tan X, Chen S, Wang X, Ye S (2016) Pirfenidone in patients with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Sci Rep 6:33226. https://doi.org/10.1038/srep33226
Liang J, Cao H, Yang Y, Ke Y, Yu Y, Sun C, Yue L, Lin J (2021) Efficacy and tolerability of nintedanib in idiopathic-inflammatory-myopathy-related interstitial lung disease: a pilot study. Front Med (Lausanne) 8:626953. https://doi.org/10.3389/fmed.2021.626953
Acknowledgements
We thank all the participants in the study.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81570058 and No. 82170076).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of Nanjing University Medical School Affiliated Drum Tower Hospital according to the policy (protocol number 2022–067-02, March 28, 2022).
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, H., Liu, H., Lyu, W. et al. An observational study of clinical recurrence in patients with interstitial lung disease related to the antisynthetase syndrome. Clin Rheumatol 42, 711–720 (2023). https://doi.org/10.1007/s10067-022-06424-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06424-4