Abstract
Background
Stachys schtschegleevii (SSC) is a herbal medicine used to treat infections. To date, this is the first study aimed to investigate the effects of SSC tea on disease activity score (DAS), serum inflammatory biomarkers and matrix metalloproteinases (MMP-1 and MMP-3) among women with rheumatoid arthritis (RA).
Methods
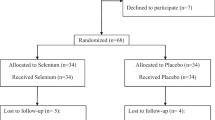
This pilot, triple-blind, randomized controlled clinical trial was conducted among forty-four women (age: 30–65 years) diagnosed with moderately active RA. Subjects were randomly assigned (1:1 ratio) into either SSC group (2.4 g/day SSC + 2.4 g/day black tea, n=22) or placebo (2.4 g/day black tea, n=22) for 8 weeks. Serum high-sensitivity C-reactive protein (hs-CRP), interleukin-1 beta (IL-1β), and MMPs were measured using ELISA. According to the American College of Rheumatology guideline considering hs-CRP, DAS28 was assessed.
Results
Both study groups had respondent rates above 94.9%. The SSC intervention caused significant reductions in the number and the percent changes of the tender joints (SSC: −74.39% vs. placebo: −57.15%, mean differences= −0.77; P<0.05) and DAS28 [SSC: −32.44% vs. placebo: −22.32%, mean differences= −0.41, P<0.05). Unlike the intervention within SSC group that showed significant reductions in the mean serum levels of hs-CRP, IL-1β, and MMP-3, SSC caused significant MMP-3 reductions (SSC: −20.59% vs. placebo: 1.29%, P<0.05).
Conclusion
The SSC intervention showed an appropriate clinical efficacy for female RA patients, accompanying remarkable reductions in the number of tender and swollen joints, DAS28, and serum levels of MMP-3. This can provide additional insights to the interventional studies controlling RA-related pathological and inflammatory outcomes.
Trial registration
Prospectively registered at the Iranian Registry of Clinical Trials (IRCT), linked to the WHO Registry Network (https://en.irct.ir/trial/11602, IRCT registration number: IRCT2015032011335N5, Registration date:2015-05-12).
Key Points • Stachys schtschegleevii improved clinical outcomes and attenuated disease severity in RA patients. • Stachys schtschegleevii ameliorated serum level of MMP-3 in RA patients. |


Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the Tabriz University of Medical Sciences (TUMS), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the TUMS. The authors had full access to all of the data in the study and take responsibility for the integrity and the accuracy of the data analysis.
Abbreviations
- SSC :
-
Stachys schtschegleevii
- RA:
-
rheumatoid arthritis
- DAS28:
-
disease activity score in 28 joints
- MMP-1:
-
matrix metalloproteinase-1
- MMP-3:
-
matrix metalloproteinase-3
- Hs-CRP:
-
high-sensitivity C-reactive protein
- IL-1β:
-
interleukin-1 beta
- FLSs:
-
fibroblast-like synoviocytes
- NF-κΒ p65:
-
nuclear factor-kappa B p65
- IRCT:
-
Iranian Registry of Clinical Trials
- BMI:
-
body mass index
- WC:
-
waist circumference
- TJC28:
-
tender joint count from 28 joints
- SJC28:
-
swollen joint count from 28 joints
- NMR:
-
nuclear magnetic resonance
- NO:
-
nitric oxide
- ELISA:
-
enzyme-linked immunosorbent assay
- TUMS:
-
Tabriz University of Medical Sciences
References
Smolen J, Aletaha D, McInnes I (2016) Rheumatoid arthritis. The Lancet 388:2023–2038. https://doi.org/10.1016/S0140-6736(16)30173-8
McInnes I, Schett G (2017) Pathogenetic insights from the treatment of rheumatoid arthritis. The Lancet 389:2328–2337. https://doi.org/10.1016/S0140-6736(17)31472-1
Shah A, St Clair EW (2015) Rheumatoid arthritis. In: Kasper DL (ed) Harrison’s principles of internal medicine, 19th edn. McGraw-Hill Education/Medical, New York, pp 2136–2149
McInnes I, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–2219. https://doi.org/10.1056/NEJMra1004965
Bustamante M, Garcia-Carbonell R, Whisenant KD, Guma M (2017) Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther 19:110. https://doi.org/10.1186/s13075-017-1303-3
Khokha R, Murthy A, Weiss A (2013) Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 13:649–665. https://doi.org/10.1038/nri3499
Tokito A, Jougasaki M (2016) Matrix metalloproteinases in non-neoplastic disorders. Int J Mol Sci 17:1178. https://doi.org/10.3390/ijms17071178
Green M, Gough A, Devlin J, Smith J, Astin P, Taylor D, Emery P (2003) Serum MMP-3 and MMP-1 and progression of joint damage in early rheumatoid arthritis. Rheumatology 42:83–88. https://doi.org/10.1093/rheumatology/keg037
Cunnane G, Fitzgerald O, Beeton C, Cawston T, Bresnihan B (2001) Early joint erosions and serum levels of matrix metalloproteinase 1, matrix metalloproteinase 3, and tissue inhibitor of metalloproteinases 1 in rheumatoid arthritis. Arthritis Rheum 44:2263–2274. https://doi.org/10.1002/1529-0131(200110)44:10%3c2263::aid-art389%3e3.0.co;2-1
Ateş A, Türkçapar N, Olmez U, Tiryaki O, Düzgün N, Uğuz E, Duman M (2007) Serum pro-matrix metalloproteinase-3 as an indicator of disease activity and severity in rheumatoid arthritis: comparison with traditional markers. Rheumatol Int 27:715–722. https://doi.org/10.1007/s00296-007-0338-1
Skacelova M, Hermanova Z, Horak P, Kazi A, Langova K (2017) Higher levels of matrix metalloproteinase-3 in patients with RA reflect disease activity and structural damage. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 161:296–302. https://doi.org/10.5507/bp.2017.015
Lerner A, Neidhöfer S, Reuter S, Matthias T (2018) MMP3 is a reliable marker for disease activity, radiological monitoring, disease outcome predictability, and therapeutic response in rheumatoid arthritis. Best Pract Res Clin Rheumatol 32:550–562. https://doi.org/10.1016/j.berh.2019.01.006
Galil SMA, El-Shafey AM, Hagrass HA, Fawzy F, Sammak AEl, (2016) Baseline serum level of matrix metalloproteinase-3 as a biomarker of progressive joint damage in rheumatoid arthritis patients. Int J Rheum Dis 19:377–384. https://doi.org/10.1111/1756-185X.12434
Houseman M, Potter C, Marshall N, Lakey R, Cawston T, Griffiths I, Young-Min S, Isaacs JD (2012) Baseline serum MMP-3 levels in patients with rheumatoid arthritis are still independently predictive of radiographic progression in a longitudinal observational cohort at 8 years follow-up. Arthritis Res Ther 14:30. https://doi.org/10.1186/ar3734
Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, Saag KG, O’dell JR, Kazi S (2012) Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res 64:640–647. https://doi.org/10.1002/acr.21649
Tomou E, Barda C, Skaltsa H (2020) Genus Stachys: a review of traditional uses, phytochemistry, and bioactivity. Medicines 7:63. https://doi.org/10.3390/medicines7100063
Rechinger KH, Hedge IC (1982) Flora Iranica. Verlagsanstalt, Graz, Akademisch Druck-u
Mozaffarian V (1996) A dictionary of Iranian plant names. Farhang Moaser, Tehran
Rezazadeh S, Kebryaeezadeh A, Pirali-Hamedani M, Shafiee A, Isfahani SG (2005) Anti-inflammatory and analgesic activity of methanolic extracts of aerial parts of Stachys schtschegleevii Sosn. and Stachys balansae Boiss. and Kotschy ex boiss in rats. Daru 13:165–169
Maleki-Dizaji N, Nazemiyeh H, Maddah N, Mehmani F, Garjani A (2008) Screening of extracts and fractions from aerial parts of Stachys schtschegleevii Sosn. for anti-inflammatory activities. Pak J Pharm Sci 21:338–343
Nazemiyeh H, Shoeb M, Movahhedin N, Kumarasamy Y, Talebpour AH, Delazar A, Nahar L, Sarker SD (2006) Phenolic compounds and their glycosides from Stachys schtschegleevii (Lamiaceae). Biochem Syst Ecol 9:721–723. https://doi.org/10.1016/j.bse.2006.05.004
Nasrollahi S, Ghoreishi M, Ebrahimabadi A, Khoobi A (2019) Gas chromatography-mass spectrometry analysis and antimicrobial, antioxidant and anti-cancer activities of essential oils and extracts of Stachys schtschegleevii plant as biological macromolecules. Int J Biol Macromol 128:718–723. https://doi.org/10.1016/j.ijbiomac.2019.01.165
Sadeghi H, Zarezade V, Sadeghi H, Toori MA, Barmak MJ, Azizi A, Ghavamizadeh M, Mostafazadeh M (2014) Anti-inflammatory activity of Stachys pilifera Benth. Iran Red Crescent Med J. https://doi.org/10.5812/ircmj.19259
Paun G, Neagu E, Moroeanu V, Albu C, Ursu TM, Zanfirescu A, Negres S, Chirita C, Radu GL (2018) Anti-inflammatory and antioxidant activities of the impatiens noli-tangere and Stachys officinalis polyphenolic-rich extracts. Rev Bras Farmacogn 28:57–64. https://doi.org/10.1016/j.bjp.2017.10.008
Barreto RS, Quintans JS, Amarante RK, Nascimento TS, Amarante RS, Barreto AS, Pereira EW, Duarte MC, Coutinho HD, Menezes IR (2016) Evidence for the involvement of TNF-α and IL-1β in the antinociceptive and anti-inflammatory activity of Stachys lavandulifolia Vahl. (Lamiaceae) essential oil and (-)-α-bisabolol, its main compound, in mice. J Ethnopharmacol 191:9–18. https://doi.org/10.1016/j.jep.2016.06.022
Benmebarek A, Zerizer S, Laggoune S, Kabouche Z (2013) Effect of Stachys mialhesi de Noé on the inflammation induced by hyperhomocysteinemia in cardiovascular diseases. Pharm Lett 5:212–223
Jalilian N, Modarresi M, Rezaie M, Ghaderi L, Bozorgmanesh M (2013) Phytotherapeutic management of polycystic ovary syndrome: role of aerial parts of wood betony (Stachys lavandulifolia). Phytother Res 27:1708–1713. https://doi.org/10.1002/ptr.4921
Chen W, Chen H, Zhu D, Luo J, Ying S, Deng Y, Yang X (2021) The clinical application value of compound Stachys sieboldii Miq granules to stable COPD patients. Minerva Med. https://doi.org/10.23736/S0026-4806.21.07188-3
Ashtiani AR, Jadidi A, Hezave AK, Safarabadi M, Pour SMA, Ghassami K, Mohammadbeigi A (2019) An analgesic effect of Stachys lavandulifolia in patients with migraine: a double-blind randomized clinical trial study. Adv Hum Biol 9:76–79. https://doi.org/10.4103/AIHB.AIHB_31_18
Sen A, Kurkcuoglu M, Bitis L, Dogan A, Baser KHC (2019) Chemical composition of endemic Stachys subnuda Montbret & Aucher ex Benth. essential oil and its anti-inflammatory and antioxidant activities. J Essent Oil Res 31:326–334. https://doi.org/10.1080/10412905.2019.1567399
Kokhdan EP, Sadeghi H, Ghafoori H, Sadeghi H, Danaei N, Javadian H, Aghamaali M (2018) Cytotoxic effect of methanolic extract, alkaloid and terpenoid fractions of Stachys pilifera against HT-29 cell line. Res Pharm Sci 13:404–412. https://doi.org/10.4103/1735-5362.236833
Abi Rizk A, Rayess YE, Iriti M, Tabet E, Mezher R, Beyrouthy ME (2020) Chemical composition, antitumor, and antioxidant effects of four Lebanese plants extracts on human pulmonary adenocarcinoma. Nat Prod Res 9:1–4. https://doi.org/10.1080/14786419.2020.1737056
Guo HF, Wang MH (2017) Anti-inflammatory and anti-cancer effect of Stachys affinis Tubers. Korean J Plant Res 30:679–685. https://doi.org/10.7732/kjpr.2017.30.6.679
Aletaha D, Neogi T, Silman A, Funovits J, Felson D, Bingham C, Birnbaum N, Burmester G, Bykerk V, Cohen M (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69:1580–1588. https://doi.org/10.1136/ard.2010.138461
Anderson JK, Zimmerman L, Caplan L, Michaud K (2011) Measures of rheumatoid arthritis disease activity: patient (PtGA) and provider (PrGA) global assessment of disease activity, disease activity score (DAS), and disease activity score with 28-joint counts (DAS28), simplified disease activity index (SDAI), clinical disease activity index (CDAI), patient activity score (PAS) and patient activity score-II (PASII), routine assessment of patient index data (RAPID), rheumatoid arthritis disease activity index (RADAI) and rheumatoid arthritis disease activity index-5 (RADAI-5), chronic arthritis systemic index (CASI), patient-based disease activity score with ESR (PDAS1) and patient-based disease activity score without ESR (PDAS2), and mean overall index for rheumatoid arthritis (MOI-RA). Arthritis Care Res 63:14–36. https://doi.org/10.1002/acr.20621
Busch CJ, Binder CJ (2017) Malondialdehyde epitopes as mediators of sterile inflammation. Biochim Biophys Acta Mol Cell Biol Lipids 1862:398–406. https://doi.org/10.1016/j.bbalip.2016.06.016
Rahzani K, Malekirad A, Zeraatpishe A, Hosseini N, Seify SMR, Abdollahi M (2013) Anti-oxidative stress activity of Stachys lavandulifolia aqueous extract in human. Cell J 14:314–317
World Medical Association (2013) World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310:2191–2194. https://doi.org/10.1001/jama.2013.281053
IPAQ Research Committee (2005) Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-short and long forms. http://www.ipaq.ki.se.
Nazemiyeh H, Maleki N, Mehmani F, Kumarasamy Y, Shoeb M, Garjani A, Sarker S (2007) Assessment of anti-inflammatory properties of ethyl acetate extract of Stachys schtschegleevii Sosn. Daru 15:174–182
Modarresi M, Hosseinzadeh L, Nematy N, Siavash-Haghighi Z, Ghanbari K (2014) Acute and subchronic toxicological evaluation of Stachys lavandulifolia aqueous extract in Wistar rats. Res Pharm Sci 9:165–172
Maleki N, Garjani A, Nazemiyeh H, Nilfouroushan N, Sadat AE, Allameh Z, Hasannia N (2001) Potent anti-inflammatory activities of hydroalcoholic extract from aerial parts of Stachys inflata on rats. J Ethnopharmacol 75:213–218. https://doi.org/10.1016/s0378-8741(01)00194-5
Ahmadzadeh A, Zabihiyeganeh M, Rahimi A, Jazayeri S (2017) The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. J Am Coll Nutr 36:9–15. https://doi.org/10.1080/07315724.2016.1140093
Haleagrahara N, Miranda-Hernandez S, Alim MA, Hayes L, Bird G, Ketheesan N (2017) Therapeutic effect of quercetin in collagen-induced arthritis. Biomed Pharmacother 90:38–46. https://doi.org/10.1016/j.biopha.2017.03.026
Ghaffari H, Ghassam BJ, Prakash HS (2012) Evaluation of antioxidant and anti-inflammatory activity of Stachys lavandulifolia. Int J Pharm Pharm Sci 4:691–696
Hayashi K, Nagamatsu T, Ito M, Hattori T, Suzuki Y (1994) Acteoside, a component of Stachys sieboldii MIQ, may be a promising antinephritic agent (2): effect of acteoside on leukocyte accumulation in the glomeruli of nephritic rats. Jpn J Pharmacol 66:47–52. https://doi.org/10.1254/jjp.66.47
Hayashi K, Nagamatsu T, Ito M, Yagita H, Suzuki Y (1996) Acteoside, a component of Stachys sieboldii MIQ, may be a promising antinephritic agent (3): effect of acteoside on expression of intercellular adhesion molecule-1 in experimental nephritic glomeruli in rats and cultured endothelial cells. Jpn J Pharmacol 70:157–168. https://doi.org/10.1254/jjp.70.157
Tam WY, Chen ZY, He ZD, Yao X, Lau CW, Huang Y (2002) Enhancement of contraction of rat mesenteric artery by acteoside: role of endothelial nitric oxide. J Nat Prod 65:990–995. https://doi.org/10.1021/np010454p
Acknowledgements
We thank all the patients who participated in the present study. This research was funded by the Research Vice-Chancellor of Tabriz University of Medical Sciences, Tabriz, Iran (registration no.: 5/4/11275).
Funding
This is a research project entitled “Effects of brewed tea of Poulk (Stachys schtschegleevii) on some biochemical parameters and disease activity in women with rheumatoid arthritis: a randomized controlled clinical trial” with registration no: 5/4/11275, approved and funded by the Connective Tissue Diseases Research Center, the Research Vice-Chancellor of Tabriz University of Medical Science, Tabriz, Iran.
Author information
Authors and Affiliations
Contributions
SP: supervised the study and contributed to the conception of the research, designed the project, and received the budget specified for this project. SP, EM, AK, HN, AE, MH, and ZS: conducted the research and data collections. SP and EM: performed statistical analyses, data interpretations, and drafting of this manuscript. All authors read and approved the final manuscript before the submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The design and procedural steps of the study were carried out according to the ethical standards of the Helsinki Declaration. The research methodology and related ethical considerations were reviewed and approved by the Ethics Committee of the Tabriz University of Medical Sciences under ethical code: 93169. Informed consent was obtained from all individual participants prior to their enrolment in the study.
Consent for publication
Not applicable.
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10067_2021_5981_MOESM3_ESM.docx
Electronic Supplementary Material 2. Scatter plots and Pearson's correlation coefficient between study biomarkers and DAS28 of RA patients at the baseline of the study. (DOCX 79 KB)
Rights and permissions
About this article
Cite this article
Mirtaheri, E., Khabbazi, A., Nazemiyeh, H. et al. Stachys schtschegleevii tea, matrix metalloproteinase, and disease severity in female rheumatoid arthritis patients: a randomized controlled clinical trial. Clin Rheumatol 41, 1033–1044 (2022). https://doi.org/10.1007/s10067-021-05981-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05981-4