Abstract
Objective
The present study was to investigate whether dihydroartemisinin (DHA), which is a highly effective and safe drug in the treatment of malaria, could be repurposed for the treatment of skin fibrosis and vascular dysfunction in systemic sclerosis (SSc).
Methods
The value of DHA was determined using a bleomycin-induced model of skin fibrosis. mRNA transcriptome analysis was performed, and the targets of DHA on fibroblasts were identified. Immunofluorescence staining was used to identify dermal vessels undergoing endothelial-to-mesenchymal transition (EndoMT). Autophagic flux was detected by western blot and mRFP-GFP-LC3 adenovirus vector transfection.
Results
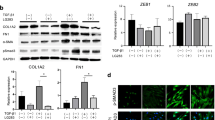
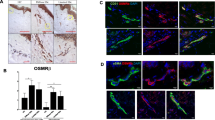
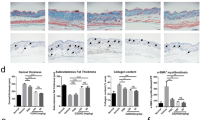
Both systemic and topical administration of DHA decreased dermal thickness and collagen deposition and alleviated EndoMT in bleomycin-induced skin fibrosis mice model. Treatment of human umbilical vein endothelial cells (HUVECs) with TGF-β1 resulted in the acquisition of the activation marker (α-SMA) and loss of endothelial markers (CD31 and VE-cadherin), a process that was restored by DHA. DHA significantly suppressed skin fibroblast activation and collagen-1 production mainly through regulating PI3K-Akt pathway. DHA also induced fibroblast autophagic flux and that autophagy dependently suppressed collagen-1 production.
Conclusion
The results of the present study revealed that oral and topical DHA administration ameliorated tissue fibrosis and protected dermal blood vessels from bleomycin-induced EndoMT. Our study has elucidated the value of repurposing DHA for the treatment of SSc.
Key Points • Oral or topical usage of DHA alleviated dermal fibrosis and EndoMT in bleomycin-induced skin fibrosis mice models. • DHA autophagy dependently inhibited fibroblast activation and collagen deposition via PI3K-ATK pathway. • DHA inhibited EndoMT of HUVECs induced by TGF-β1 by the downregulation of α-SMA and the upregulation of CD31 and VE-cadherin. |





Similar content being viewed by others
Data availability
The transcriptome data, including processed matrix and raw data, have been uploaded to the GEO database, and the index number is GSE162550.
References
Poudel DR, Derk CT (2018) Mortality and survival in systemic sclerosis: a review of recent literature. Curr Opin Rheumatol 30:588–593. https://doi.org/10.1097/bor.0000000000000551
Denton CP, Khanna D (2017) Systemic sclerosis. Lancet 390:1685–1699. https://doi.org/10.1016/s0140-6736(17)30933-9
Korman B (2019) Evolving insights into the cellular and molecular pathogenesis of fibrosis in systemic sclerosis. Transl Res 209:77–89. https://doi.org/10.1016/j.trsl.2019.02.010
Cutolo M, Soldano S, Smith V (2019) Pathophysiology of systemic sclerosis: current understanding and new insights. Expert Rev Clin Immunol 15:753–764. https://doi.org/10.1080/1744666x.2019.1614915
Lu X, Gong J, Dennery PA, Yao H (2019) Endothelial-to-mesenchymal transition: pathogenesis and therapeutic targets for chronic pulmonary and vascular diseases. Biochem Pharmacol 168:100–107. https://doi.org/10.1016/j.bcp.2019.06.021
Manetti M, Romano E, Rosa I, Guiducci S, Bellando-Randone S, De Paulis A, Ibba-Manneschi L, Matucci-Cerinic M (2017) Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann Rheum Dis 76:924–934. https://doi.org/10.1136/annrheumdis-2016-210229
Kitao A, Sato Y, Sawada-Kitamura S, Harada K, Sasaki M, Morikawa H, Shiomi S, Honda M, Matsui O, Nakanuma Y (2009) Endothelial to mesenchymal transition via transforming growth factor-beta1/Smad activation is associated with portal venous stenosis in idiopathic portal hypertension. Am J Pathol 175:616–626. https://doi.org/10.2353/ajpath.2009.081061
Gutman J, Kovacs S, Dorsey G, Stergachis A, Ter Kuile FO (2017) Safety, tolerability, and efficacy of repeated doses of dihydroartemisinin-piperaquine for prevention and treatment of malaria: a systematic review and meta-analysis. Lancet Infect Dis 17:184–193. https://doi.org/10.1016/s1473-3099(16)30378-4
Zhang F, Ma Q, Xu Z, Liang H, Li H, Ye Y, Xiang S, Zhang Y, Jiang L, Hu Y, Wang Z, Wang X, Zhang Y, Gong W, Liu Y (2017) Dihydroartemisinin inhibits TCTP-dependent metastasis in gallbladder cancer. J Exp Clin Cancer Res 36:68. https://doi.org/10.1186/s13046-017-0531-3
Yang DX, Qiu J, Zhou HH, Yu Y, Zhou DL, Xu Y, Zhu MZ, Ge XP, Li JM, Lv CJ, Zhang HQ, Yuan WD (2018) Dihydroartemisinin alleviates oxidative stress in bleomycin-induced pulmonary fibrosis. Life Sci 205:176–183. https://doi.org/10.1016/j.lfs.2018.05.022
Lin R, Zhang Z, Chen L, Zhou Y, Zou P, Feng C, Wang L, Liang G (2016) Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett 381:165–175. https://doi.org/10.1016/j.canlet.2016.07.033
Kalani K, Chaturvedi V, Trivedi P, Tondon S, Srivastava SK (2019) Dihydroartemisinin and its analogs: a new class of antitubercular agents. Curr Top Med Chem 19:594–599. https://doi.org/10.2174/1568026619666190304142802
Li L, Chen X, Dong F, Liu Q, Zhang C, Xu D, Allen TD, Liu J (2018) Dihydroartemisinin up-regulates VE-cadherin expression in human renal glomerular endothelial cells. J Cell Mol Med 22:2028–2032. https://doi.org/10.1111/jcmm.13448
Frech T, De Domenico I, Murtaugh MA, Revelo MP, Li DY, Sawitzke AD, Drakos S (2014) Autophagy is a key feature in the pathogenesis of systemic sclerosis. Rheumatol Int 34:435–439. https://doi.org/10.1007/s00296-013-2827-8
Dumit VI, Küttner V, Käppler J, Piera-Velazquez S, Jimenez SA, Bruckner-Tuderman L, Uitto J, Dengjel J (2014) Altered MCM protein levels and autophagic flux in aged and systemic sclerosis dermal fibroblasts. J Invest Dermatol 134:2321–2330. https://doi.org/10.1038/jid.2014.69
Sosulski ML, Gongora R, Danchuk S, Dong C, Luo F, Sanchez CG (2015) Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFβ1. Aging Cell 14:774–783. https://doi.org/10.1111/acel.12357
Wang L, Li J, Shi X, Li S, Tang PM, Li Z, Li H, Wei C (2019) Antimalarial dihydroartemisinin triggers autophagy within HeLa cells of human cervical cancer through Bcl-2 phosphorylation at Ser70. Phytomedicine 52:147–156. https://doi.org/10.1016/j.phymed.2018.09.221
Thongchot S, Vidoni C, Ferraresi A, Loilome W, Yongvanit P, Namwat N, Isidoro C (2018) Dihydroartemisinin induces apoptosis and autophagy-dependent cell death in cholangiocarcinoma through a DAPK1-BECLIN1 pathway. Mol Carcinog 57:1735–1750. https://doi.org/10.1002/mc.22893
Zhang Z, Yao Z, Zhao S, Shao J, Chen A, Zhang F, Zheng S (2017) Interaction between autophagy and senescence is required for dihydroartemisinin to alleviate liver fibrosis. Cell Death Dis 8:e2886. https://doi.org/10.1038/cddis.2017.255
Liu J, Ren Y, Hou Y, Zhang C, Wang B, Li X, Sun R, Liu J (2019) Dihydroartemisinin induces endothelial cell autophagy through suppression of the Akt/mTOR pathway. J Cancer 10:6057–6064. https://doi.org/10.7150/jca.33704
Piera-Velazquez S, Li Z, Jimenez SA (2011) Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol 179:1074–1080. https://doi.org/10.1016/j.ajpath.2011.06.001
Funding
This work was supported by the National Natural Science Foundation of China (81974251 and 81801593).
Author information
Authors and Affiliations
Contributions
Rui Li and Hanlin Yin contributed equally to this work.
Corresponding authors
Ethics declarations
Ethical statement
Sample collection and written informed consent were approved by the ethics committee of Shanghai Jiao Tong University School of Medicine that is affiliated with Renji Hospital.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PNG 5238 kb)
Rights and permissions
About this article
Cite this article
Li, R., Yin, H., Wang, J. et al. Dihydroartemisinin alleviates skin fibrosis and endothelial dysfunction in bleomycin-induced skin fibrosis models. Clin Rheumatol 40, 4269–4277 (2021). https://doi.org/10.1007/s10067-021-05765-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05765-w