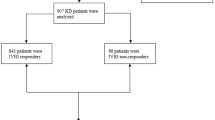Abstract
Introduction/objectives
The dosing of intravenous immunoglobulin (IVIG) therapy for Kawasaki disease (KD) has been a matter of debate for decades, with recent studies implicating that larger doses lead to better outcomes. Despite this, few have investigated post-IVIG infusion immunoglobulin G (IgG) levels in relation to outcomes of KD such as response to IVIG and development of coronary artery abnormalities (CAAs). The present study investigated how varying levels of post-infusion IgG affected these outcomes.
Method
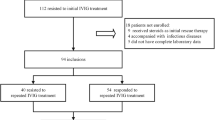
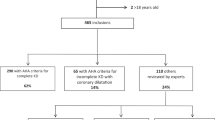
We collected demographic and laboratory data, including post-infusion IgG, from children with KD who were admitted to six hospitals in Japan between 2006 and 2012. We conducted multivariate analyses to examine the relationship between independent variables and non-response to IVIG and development of CAAs. We used random forest, a decision tree-based machine learning tool, to investigate the marginal effect of varying post-infusion IgG levels on non-response to IVIG and development of CAAs.
Results
Of 456 patients included in the study, 130 (28.5%) were non-responders and 38 (8.3%) developed CAAs. Sodium, post-infusion IgG, and AST were significantly associated with non-response. Post-infusion IgG and sodium were significantly associated with CAA development. The random forest plots revealed a decrease in non-response and CAA rates with increasing post-infusion IgG until post-infusion IgG was near the median (2821 mg/dL), after which the non-response and CAA rates leveled off.
Conclusions
Greater post-infusion IgG is associated with better response to IVIG and decreased CAA development in KD patients, but this effect levels off at post-infusion IgG levels greater than the median.
Key points • Though previous studies have shown that post-intravenous immunoglobulin (IVIG) infusion immunoglobulin G (IgG) is associated with non-response to IVIG therapy and coronary artery abnormality (CAA) development in Kawasaki disease (KD) patients, no study has investigated the relationship between varying levels of post-infusion IgG and these clinical outcomes. • Our study showed that non-response to IVIG therapy and CAA development in Kawasaki disease patients follow a decreasing trend with increasing post-infusion IgG at post-infusion IgG levels below the median. • At values of post-infusion IgG greater than the median, non-response and CAA development rates remain relatively constant with increasing post-infusion IgG. • Our study suggests that when post-infusion IgG is greater than the median, IgG may have fully bound to the therapeutic targets of KD, and in these patients, there may be limited benefit in administering additional IVIG. |




Similar content being viewed by others
References
Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, Tamura T, Hirose O, Manabe Y, Yokoyama T (1984) High-dose intravenous gammaglobulin for Kawasaki disease. Lancet 2(8411):1055–1058. https://doi.org/10.1016/s0140-6736(84)91504-6
McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E, American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention (2017) Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 135(17):e927–e999. https://doi.org/10.1161/CIR.0000000000000484
Nakagama Y, Inuzuka R, Hayashi T, Shindo T, Hirata Y, Shimizu N, Inatomi J, Yokoyama Y, Namai Y, Oda Y, Takamizawa M, Harita Y, Oka A (2016) Fever pattern and C-reactive protein predict response to rescue therapy in Kawasaki disease. Pediatr Int 58(3):180–184. https://doi.org/10.1111/ped.12762
Iwashima S, Kimura M, Ishikawa T, Ohzeki T (2011) Importance of C-reactive protein level in predicting non-response to additional intravenous immunoglobulin treatment in children with Kawasaki disease: a retrospective study. Clin Drug Investig 31(3):191–199. https://doi.org/10.2165/11538910-000000000-00000
Kobayashi T, Kobayashi T, Morikawa A, Ikeda K, Seki M, Shimoyama S, Ishii Y, Suzuki T, Nakajima K, Sakamoto N, Arakawa H (2013) Efficacy of intravenous immunoglobulin combined with prednisolone following resistance to initial intravenous immunoglobulin treatment of acute Kawasaki disease. J Pediatr 163(2):521–526. https://doi.org/10.1016/j.jpeds.2013.01.022
Furukawa T, Kishiro M, Akimoto K, Nagata S, Shimizu T, Yamashiro Y (2008) Effects of steroid pulse therapy on immunoglobulin-resistant Kawasaki disease. Arch Dis Child 93:142–146. https://doi.org/10.1136/adc.2007.126144
Suzuki H, Terai M, Hamada H, Honda T, Suenaga T, Takeuchi T, Yoshikawa N, Shibuta S, Miyawaki M, Oishi K, Yamaga H, Aoyagi H, Iwahashi S, Miyashita R, Onouchi Y, Sasago K, Suzuki Y, Hata A (2011) Cyclosporin A treatment for Kawasaki disease refractory to initial and additional intravenous immunoglobulin. Pediatr Infect Dis J 30(10):871–876. https://doi.org/10.1097/INF.0b013e318220c3cf
Burns JC, Best BM, Mejias A, Mahony L, Fixler DE, Jafri HS, Melish ME, Jackson MA, Asmar BI, Lang DJ, Connor JD, Capparelli EV, Keen ML, Mamun K, Keenan GF, Ramilo O (2008) Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr 153:833–838. https://doi.org/10.1016/j.jpeds.2008.06.011
Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, Kobayashi T, Morikawa A (2006) Prediction of intravenous immunoglobulin non-response in patients with Kawasaki disease. Circulation 113:2606–2612. https://doi.org/10.1161/CIRCULATIONAHA.105.592865
Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, Matuishi T (2006) Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr 149:237–240. https://doi.org/10.1016/j.jpeds.2006.03.050
Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, Kogaki S, Hara J (2007) Prediction of non-responsiveness to standard high-dose γ-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr 166:131–137. https://doi.org/10.1007/s00431-006-0223-z
Yamazaki-Nakashimada MA, Gámez-González LB, Murata C, Honda T, Yasukawa K, Hamada H (2019) IgG levels in Kawasaki disease and its association with clinical outcomes. Clin Rheumatol 38(3):749–754. https://doi.org/10.1007/s10067-018-4339-0
Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode MP, Mason WH, Meissner HC, Rowley AH, Shulman ST, Reddy V, Sundel RP, Wiggins JW, Colton T, Melish ME, Rosen FS (1991) A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med 324(23):1633–1639. https://doi.org/10.1056/NEJM199106063242305
Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, Kato T, Hara T, Hamaoka K, Ogawa S, Miura M, Nomura Y, Fuse S, Ichida F, Seki M, Fukazawa R, Ogawa C, Furuno K, Tokunaga H, Takatsuki S, Hara S, Morikawa A, RAISE study group investigators (2012) Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet 379(9826):1613–20.1. https://doi.org/10.1016/S0140-6736(11)61930-2
Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, Ishii M, Harada K, Kawasaki Disease Research Committee (2005) Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int 47(2):232–234. https://doi.org/10.1111/j.1442-200x.2005.02033.x
Hastie T, Tibshirani R, Friedman JH (2001) The elements of statistical learning. Springer-Verlag, New York
Lo MS, Newburger JW (2018) Role of intravenous immunoglobulin in the treatment of Kawasaki disease. Int J Rheum Dis 21(1):64–69. https://doi.org/10.1111/1756-185X.13220
Makowsky R, Wiener HW, Ptacek TS, Silva M, Shendre A, Edberg JC, Portman MA, Shrestha S (2013) FcγR gene copy number in Kawasaki disease and intravenous immunoglobulin treatment response. Pharmacogenet Genomics 23(9):455–462. https://doi.org/10.1097/FPC.0b013e328363686e
Bonilla FA (2008) Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. Immunol Allergy Clin North Am 28(4):803–819. https://doi.org/10.1016/j.iac.2008.06.006
Eigenmann MJ, Karlsen TV, Krippendorff BF, Tenstad O, Fronton L, Otteneder MB, Wiig H (2017) Interstitial IgG antibody pharmacokinetics assessed by combined in vivo- and physiologically-based pharmacokinetic modelling approaches. J Physiol 595(24):7311–7330. https://doi.org/10.1113/JP274819
Oates-Whitehead RM, Baumer JH, Haines L, Love S, Maconochie IK, Gupta A, Roman K, Dua JS, Flynn I (2003) Intravenous immunoglobulin for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev 2003(4):CD004000. https://doi.org/10.1002/14651858.CD004000
Baba R, Shibata A, Tsurusawa M (2005) Single high-dose intravenous immunoglobulin therapy for Kawasaki disease increases plasma viscosity. Circ J 69(8):962–964. https://doi.org/10.1253/circj.69.962
Wada Y, Kamei A, Fujii Y, Ishikawa K, Chida S (2006) Cerebral infarction after high-dose intravenous immunoglobulin therapy for Kawasaki disease. J Pediatr 148(3):399–400. https://doi.org/10.1016/j.jpeds.2005.10.027
Nolan BE, Wang Y, Pary PP, Luban NLC, Wong ECC, Ronis T (2018) High-dose intravenous immunoglobulin is strongly associated with hemolytic anemia in patients with Kawasaki disease. Transfusion 58(11):2564–2571. https://doi.org/10.1111/trf.14879
Kawamura Y, Takeshita S, Kanai T, Yoshida Y, Nonoyama S (2016) The combined usefulness of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in predicting intravenous immunoglobulin resistance with Kawasaki disease. J Pediatr 178:281–284.e1. https://doi.org/10.1016/j.jpeds.2016.07.035
Acknowledgments
We would like to thank Dr. Hajime Nishimoto at Saitama Citizens Medical Center, Dr. Yusuke Shiozawa at Nippon Medical School, and Dr. Hiroki Kitaoka at Yaizu City Hospital for their assistance in data collection.
Availability of data and material
Data used in this study is available upon reasonable request.
Code availability
The coding for the data analysis used in this study is available upon reasonable request.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Ryunosuke Goto performed the data analysis and wrote the manuscript. Ryo Inuzuka directed the research. Data collection was performed by Ryo Inuzuka, Takahiro Shindo, Yoshiyuki Namai, Yoichiro Oda, Yutaka Harita, and Akira Oka. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Institutional Review Board at the University of Tokyo (IRB number: 10103-(1)).
Consent to participate
Passive parental consent (opt-out consent) for the retrospective use of data was obtained for all participants.
Consent for publication
Passive parental consent (opt-out consent) for publication of this study was obtained for all participants.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goto, R., Inuzuka, R., Shindo, T. et al. Relationship between post-IVIG IgG levels and clinical outcomes in Kawasaki disease patients: new insight into the mechanism of action of IVIG. Clin Rheumatol 39, 3747–3755 (2020). https://doi.org/10.1007/s10067-020-05153-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05153-w