Abstract
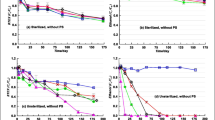
Enhanced bioremediation combined with in-situ chemical oxidation has the potential to remediate groundwater contaminated with organics. To explore the remediating effects of these two approaches and to evaluate their combined feasibility, traditional gasoline (no ethanol) and ethanol-gasoline (10% ethanol, v/v) were released into experimental sand tanks (TG-tank and EG-tank, respectively) under the same water-flow conditions. Nitrate and sulfate were added to enhance bioremediation and then persulfate was injected to encourage chemical oxidation. Two push–pull tests, using persulfate and bromide respectively, were conducted to compare their behavior. The results showed that nitrate reduction, rather than sulfate reduction, enhanced BTEX (benzene, toluene, ethylbenzene, and xylene) biodegradation, but the presence of ethanol inhibited these processes. The detected concentration of BTEX in the TG-tank was lower than that in the EG-tank, and the first-order decay rate constants of BTEX in the TG-tank and EG-tank under nitrate-adjusted conditions were 0.0058 and 0.0016 d−1, respectively. The first persulfate injection (10 g L−1) resulted in 86 and 94% concentration decreases of BTEX in the TG-tank and EG-tank, respectively, at first-order decay rates of 0.0180 and 0.0181 d−1, respectively. However, the subsequent persulfate injections at 20 and 50 g L−1 had no significant removal effect on BTEX. Persulfate oxidation made pH decrease (but it quickly recovered) and did not significantly inhibit nitrate reduction. This study suggests that enhanced nitrate reduction can be combined with persulfate oxidation for the in-situ remediation of groundwater contaminated by petroleum hydrocarbons.
Résumé
La bio remédiation optimisée par une combinaison avec de l’oxydation chimique in-situ permet potentiellement de remédier à la contamination des eaux souterraines par des organiques. Pour explorer les effets de remédiation de ces deux approches et pour évaluer la possibilité de les combiner, de l’essence classique (sans éthanol) et de l’essence avec éthanol (10% éthanol, v/v) ont été déversées dans des cuves expérimentales remplie de sable (respectivement cuve TG et cuve EG). Du nitrate et sulfate ont été ajoutés afin d’augmenter la bio remédiation et du persulfate a été injecté pour faciliter l’oxydation chimique. Deux tests “push–pull”, utilisant respectivement du persulfate et du bromure, ont été menés afin de comparer les comportements. Les résultats montrent que la réduction du nitrate, plus que la réduction du sulfate, augmente la biodégradation des BTEX (benzène, toluène, éthylbenzène, et xylène). Les concentrations détectées de BTEX dans la cuve TG sont inférieures à celles de la cuve EG, et les taux de décomposition de premier ordre du BTEX dans la cuve TG et la cuve EG sous conditions ajustées par le nitrate sont respectivement de 0.0058 et 0.0016 j–1. La première injection de persulfate (10 g L–1) amène une réduction des concentrations de 86 et 94% de BTEX respectivement dans les cuves TG et EG avec un taux de décroissance de première ordre respectivement de 0.0180 à 0.0181 j–1. Cependant, les injections suivantes de persulfate à 20 et 50 g L–1 n’ont pas d’effet significatif sur la suppression du BTEX. L’oxydation du persulfate entraine une diminution du pH (mais le retour à la valeur initiale se fait rapidement) et ne limite pas significativement la réduction du nitrate. Cette étude suggère que l’augmentation de la réduction du nitrate peut être combine avec l’oxydation du persulfate pour la remédiation in-situ d’eaux souterraines contaminées par des hydrocarbures pétroliers.
Resumen
La biorremediación combinada con la oxidación química in situ tiene el potencial de remediar las aguas subterráneas contaminadas con sustancias orgánicas. Para analizar los efectos remediadores de estos dos procedimientos y evaluar su viabilidad combinada, se vertieron gasolina tradicional (sin etanol) y etanol-gasolina (10% de etanol, v/v) en tanques de arena experimentales (tanque TG y tanque EG, respectivamente) bajo las mismas condiciones de flujo de agua. Se añadieron nitrato y sulfato para mejorar la biorremediación y luego se inyectó persulfato para fomentar la oxidación química. Se realizaron dos pruebas push–pull, utilizando persulfato y bromuro respectivamente, para comparar su comportamiento. Los resultados mostraron que la reducción del nitrato, más que la del sulfato, potenció la biodegradación de BTEX (benceno, tolueno, etilbenceno y xileno), pero la presencia de etanol inhibió estos procesos. La concentración detectada de BTEX en el tanque TG fue inferior a la del tanque EG, y las constantes de velocidad de decaimiento de primer orden de los BTEX en el tanque TG y en el tanque EG en condiciones ajustadas a los nitratos fueron de 0.0058 y 0.0016 d–1, respectivamente. La primera inyección de persulfato (10 g L–1) dio lugar a una disminución de la concentración de BTEX del 86% y el 94% en el tanque TG y el tanque EG, respectivamente, con tasas de decaimiento de primer orden de 0.0180 y 0.0181 d–1, respectivamente. Sin embargo, las inyecciones posteriores de persulfato a 20 y 50 g L–1 no tuvieron un efecto significativo de eliminación de BTEX. La oxidación con persulfato hizo que el pH disminuyera (pero se recuperó rápidamente) y no inhibió significativamente la reducción de nitratos. Este estudio sugiere que la reducción de nitrato puede combinarse con la oxidación de persulfato para la remediación in situ de aguas subterráneas contaminadas por hidrocarburos de petróleo.
摘要
增强型生物修复联合原位化学氧化具有修复受有机物污染地下水的潜力。为了探索这两种方法的修复效果并评估其联合可行性, 在相同水流条件下, 将传统汽油(无乙醇)和乙醇汽油(10% 乙醇, v/v)加入到实验砂槽中(分别为TG-tank 和 EG-tank)。加入硝酸盐和硫酸盐用以增强生物修复, 投注过硫酸盐以促进化学氧化。本研究分别采用过硫酸盐和溴化物的注-提实验来比较其行为。结果表明, 是硝酸盐还原而不是硫酸盐还原增强了 BTEX(苯、甲苯、乙苯和二甲苯)的生物降解, 但乙醇的存在会抑制该过程。TG-tank中BTEX的检测浓度低于EG-tank中的, 在硝酸盐调节条件下, TG-tank和EG-tank中BTEX的一级衰减速率常数分别为0.0058 d–1和 0.0016 d–1。第一次过硫酸盐的投注(10 g L–1)导致 TG 罐和 EG 罐中 BTEX 浓度分别下降 86% 和 94%, 一级衰减速率常数分别为 0.0180 和 0.0181 d–1。然而, 随后以 20 和 50 g L–1 投注过硫酸盐对 BTEX 没有显著去除效果。过硫酸盐氧化使 pH 值降低(但很快恢复)并且不会显著抑制硝酸盐还原。这项研究表明, 增强型硝酸盐还原可以与过硫酸盐氧化相结合, 用于原位修复被石油烃污染的地下水。
Resumo
A biorremediação aumentada combinada com a oxidação química in-situ tem potencial para remediar águas subterrâneas contaminadas com substâncias orgânicas. Para explorar os efeitos remediadores destas duas abordagens e para avaliar a viabilidade de sua combinação, gasolina tradicional (sem etanol) e gasolina com etanol (10% etanol, v/v) foram liberadas em tanques experimentais de areia (tanque TG e tanque EG, respectivamente) sob as mesmas condições de fluxo de água. Nitrato e sulfato foram adicionados para melhorar a biorremediação, e então o persulfato foi injetado para estimular a oxidação química. Ensaios de push–pull, usando persulfato e brometo respectivamente, foram conduzidos para comparar seus comportamentos. Os resultados mostram que a redução por nitrato, mais do que a redução por sulfato, aumentou a biodegradação de BTEX (benzeno, tolueno, etilbenzeno e xileno), mas a presença do etanol inibiu esses processos. A concentração detectada de BTEX no tanque TG era menor do que no tanque EG, e as taxas constantes de decaimento de primeira ordem de BTEX nos tanques TG e EG sob condições ajustadas por nitrato eram de 0.0058 e 0.0016 d–1, respectivamente. A primeira injeção de persulfato (10 g L–1) resultou na diminuição em 86 e 94% das concentrações de BTEX nos tanques TG e EG, respectivamente, a taxas de decaimento de primeira ordem de 0.0180 e 0.0181 d–1, respectivamente. Entretanto, as subsequentes injeções de persulfato a 20 e 50 g L–1 não tiveram efeitos significativos sobre a remoção de BTEX. A oxidação por persulfato provocou diminuição do pH (embora rapidamente recuperado) e não inibiu significativamente a redução por nitrato. Este estudo sugere que a redução aumentada por nitrato pode ser combinada com oxidação por persulfato para a remediação in-situ de águas subterrâneas contaminadas por hidrocarbonetos de petróleo.






Similar content being viewed by others
Change history
09 December 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10040-021-02432-x
References
Azubuike CC, Chikere CB, Okpokwasili GC (2016) Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects. World J Microbiol Biotechnol 32(11):180
Barbora T, Barbora T, Kamil N, Josef D, Ondrej N, Petr H (2007) Geochemical heterogeneity and isotope geochemistry of natural attenuation processes in a gasoline-contaminated aquifer at the Hnevice site, Czech Republic. Hydrogeol J 15(5):961–976
Barker JF, Patrick GC, Major D (1987) Natural attenuation of aromatic hydrocarbons in a shallow sand aquifer. Ground Water Monit Remediat 7(1):64–71
Bartlett CK, Slawson RM, Thomson NR (2019) Response of sulfate-reducing bacteria and supporting microbial community to persulfate exposure in a continuous flow system. Environ Sci-Proc Imp 21(7):1193–1203
Beyer C, Bauer S, Kolditz O (2006) Uncertainty assessment of contaminant plume length estimates in heterogeneous aquifers. J Contam Hydrol 87(1):73–95
Bockelmann A, Zamfirescu D, Ptak T, Grathwohl P, Teutsch G (2003) Quantification of mass fluxes and natural attenuation rates at an industrial site with a limitedmonitoring network: a case study. J Contam Hydrol 60(1–2):97–121
Chen YD, Barker JF, Gui L (2008) A strategy for aromatic hydrocarbon bioremediation under anaerobic conditions and the impacts of ethanol: a microcosm study. J Contam Hydrol 96:17–31
Chen YD, Jiang YP, Zhu YN, Xia Y, Cheng YP, Huang YQ, Liu HL (2013) Fate and transport of ethanol-blended dissolved BTEX hydrocarbons: a quantitative tracing study of a sand tank experiment. Environ Earth Sci 70(1):49–56
Chen YD, Cheng YP, Jiang YP, Yan YN, Liu ZT, Tong QL, Sun ZS (2017) Pump-and-treat method to remove nitrate from groundwater with liquor as the carbon source. Environ Earth Sci 76:392
Chen L, Hu X, Cai T, Yang Y, Zhao R, Liu C, Li A, Jiang C (2019) Degradation of Triclosan in soils by thermally activated persulfate under conditions representative of in situ chemical oxidation (ISCO). Chem Eng J 369:344–352
Chen YD, He LW, Xia Y, Cheng YP, Jiang YP (2020) Comparison of BTEX attenuation behaviors between ethanol gasoline and tradition gasoline in contaminated groundwater (in Chinese). Acta Sci Circumst 40:2142–2149
Cheng YP, Chen YD, Jiang YP, Jiang LZ, Sun LQ, Li LY, Huang JY (2016) Migration of BTEX and biodegradation in shallow underground water through fuel leak simulation. BioMed Res Int 2016:1–8. https://doi.org/10.1155/2016/7040872
Corseuil HX, Gomez DE, Schambeck CM, Ramos DT, Alvarez PJ (2015) Nitrate addition to groundwater impacted by ethanol-blended fuel accelerates ethanol removal and mitigates the associated metabolic flux dilution and inhibition of BTEX biodegradation. J Contam Hydrol 174:1–9
Cunningham JA, Rahme H, Hopkins GD, Carmen L, Martin R (2001) Enhanced in situ bioremediation of BTEX-contaminated groundwater by combined injection of nitrate and sulfate. Environ Sci Technol 35(8):1663–1670
Da Silva MLB, Corseuil HX (2012) Groundwater microbial analysis to assess enhanced BTEX biodegradation by nitrate injection at a gasohol-contaminated site. Int Biodeterior Biodegradation 67:21–27
Farhadian M, Vachelard C, Duchez D, Larroche C (2008) In situ bioremediation of monoaromatic pollutants in groundwater: a review. Bioresour Technol 99(13):5296–5308
Fedrizzi F, Ramos DT, Lazzarin HS, Fernandes M, Larose C, Vogel TM, Corseuil HX (2017) A modified approach for in situ chemical oxidation coupled to biodegradation enhances light nonaqueous phase liquid source-zone remediation. Environ Sci Technol 51(1):463–472
Jiang YP, Chen YD, Zhang Y, Huang YQ (2010) Effects of ethanol nitrate-enhanced bioremediation on permeability of porous media (in Chinese). Geol J China Univ 16(1):32–38
Jiang FC, Li YL, Zhou W, Yang S, Yang Z, Ning Y, Liu DQ, Zhang Y, Yang BG, Tang Z (2021) Cysteine enhanced degradation of monochlorobenzene in groundwater by ferrous iron persulfate process: impacts of matrix species and toxicity evaluation in ISCO. Chemosphere 271:129520
Kolhatkar R, Schnobrich M (2017) Land application of sulfate salts for enhanced natural attenuation of benzene in groundwater: a case study. Ground Water Monit Remediat 37(2):43–57
Krembs FJ, Siegrist RL, Crimi ML, Furrer RF, Petri BG (2010) ISCO for groundwater remediation: analysis of field applications and performance. Ground Water Monit Remediat 30(4):42–53
Liang C, Huang CF, Chen YJ (2008) Potential for activated persulfate degradation of BTEX contamination. Water Resour 42(15):4091–4100
Ma J, Yang Y, Jiang X, Xie ZT, Li XX, Chen CZ, Chen HK (2018) Impacts of inorganic anions and natural organic matter on thermally activated persulfate oxidation of BTEX in water. Chemosphere 190:296–306
Nicholls HCG, Mallinson HEH, Rolfe SA, Hjort M, Spence MJ, Thornton SF (2020) Influence of contaminant exposure on the development of aerobic ETBE biodegradation potential in microbial communities from a gasoline-impacted aquifer. J Hazard Mater 388:122022
O’Connor D, Hou D, Ok YS, Song Y, Sarmah AK, Li X, Tack FM (2018) Sustainable in situ remediation of recalcitrant organic pollutants in groundwater with controlled release materials: a review. J Control Release 283:200–213
Ostad-Ali-Askari K, Shayannejad M (2021) Quantity and quality modelling of groundwater to manage water resources in Isfahan-Borkhar aquifer. Environ Dev Sustain 23(11):1–17
Ostad-Ali-Askari K, Shayannejad M, Ghorbanizadeh-Kharazi H (2016) Artificial neural network for modeling nitrate pollution of groundwater in marginal area of Zayandeh-Rood River, Isfahan, Iran. KSCE J Civ Eng 21(1):134–140
Ostad-Ali-Askari K, Ghorbanizadeh KH, Shayannejad M, Zareian MJ (2019) Effect of management strategies on reducing negative impacts of climate change on water resources of the Isfahan-Borkhar aquifer using MODFLOW. River Res Appl 35(6):611–631
Ostad-Ali-Askari K, Ghorbanizadeh KH, Shayannejad M, Zareian MJ (2020) Effect of climate change on precipitation patterns in an arid region using GCM models: case study of Isfahan-Borkhar plain. Nat Hazards Rev 21(2):04020006
Palma E, Espinoza TA, Daghio M, Franzetti A, Tsiota P, Cruz VC, Papini MP (2019) Bioelectrochemical treatment of groundwater containing BTEX in a continuous-flow system: substrate interactions, microbial community analysis, and impact of sulfate as a co-contaminant. New Biotech 53:41–48
Pieter J, Richard L, Ilse VK, Johan P, Jan B, Marjan J, Jan B, Florimond DS (2010) Containment of groundwater pollution (methyl tertiary butyl ether and benzene) to protect a drinking-water production site in Belgium. Hydrogeol J 18(8):1917–1925
Ponsin V, Coulomb B, Guelorget Y, Maier J, Hhener P (2014) In situ biostimulation of petroleum hydrocarbon degradation by nitrate and phosphate injection using a dipole well configuration. J Contam Hydrol 171:22–31
Rama F, Ramos DT, Muller JB, Corseuil HX, Miotliński K (2019) Flow field dynamics and high ethanol content in gasohol blends enhance BTEX migration and biodegradation in groundwater. J Contam Hydrol 222:17–30
Rivett MO, Buss SR, Morgan P, Smith JW, Bemment CD (2008) Nitrate attenuation in groundwater: a review of biogeochemical controlling processes. Water Resour 42:4215–4232
Santos A, Fernandez J, Rodriguez S, Dominguez CM, Lominchar MA, Lorenzo D, Romero A (2018) Abatement of chlorinated compounds in groundwater contaminated by HCH wastes using ISCO with alkali activated persulfate. Sci Total Environ 615:1070–1077
Satapanajaru T, Chokejaroenrat C, Sakulthaew C, Yoo-lam M (2017) Remediation and restoration of petroleum hydrocarbon containing alcohol-contaminated soil by persulfate oxidation activated with soil minerals. Water Air Soil Pollut 228:345
Shayan M, Thomson NR, Aravena R, Barker JF, Madsen EL, Marchesi M, DeRito CM, Bouchard D, Buscheck T, Kolhatkar R, Daniels EJ (2018) Integrated plume treatment using persulfate coupled with microbial sulfate reduction. Ground Water Monit Remediat 38:45–61
Sra KS, Thomson NR, Barker JF (2010) Persistence of persulfate in uncontaminated aquifer materials. Environ Sci Technol 44:3098–3104
Sra KS, Neil R, Thomson MA, Barker AJF (2013a) Persulfate treatment of dissolved gasoline compounds. J Hazard Toxic Radioact Waste 17:9–15
Sra KS, Thomson NR, Barker JF (2013b) Persulfate injection into a gasoline source zone. J Contam Hydrol 2013(150):35–44
Steiner LV, Débora TR, Liedke AMR, Serbent MP, Corseuil HX (2018) Ethanol content in different gasohol blend spills influences the decision-making on remediation technologies. J Environ Manag 212:8–16
Sun LQ, Chen YD, Jiang LZ, Cheng YP (2018) Numerical simulation of the effect about groundwater level fluctuation on the concentration of BTEX dissolved into source zone. IOP Conf Ser Earth Environ Sci 111(1):012017
Suthersan S, McDonough J, Schnobrich M, Divine C (2017) In situ chemical treatment a love-hate relationship. Ground Water Monit Remediat 37(1):17–26
Sutton NB, Grotenhuis JTC, Langenhoff AAM, Rijnaarts HM (2011) Efforts to improve coupled in situ chemical oxidation with bioremediation a review of optimization strategies. J Soils Sediments 11:129–140
Sutton NB, Kalisz M, Krupanek J, Marek J, Grotenhuis T, Smidt H, de Weert J, Rijnaarts HH, van Gaans P, Keijzer T (2014) Geochemical and microbiological characteristics during in situ chemical oxidation and in situ bioremediation at a diesel contaminated site. Environ Sci Technol 48:2352–2360
Tsitonaki A, Petri B, Crimi M, Mosbk H, Siegrist RL, Bjerg PL (2010) In situ chemical oxidation of contaminated soil and groundwater using persulfate: a review. Crit Rev Environ Sci Technol 40(1):55–91
Wiedemeier TH, Wilson JH, Kampbell DH (1999) Technical protocol for implementing intrinsic remediation with long-term monitoring for natural attenuation of fuel contamination dissolved in groundwater, vol 1. Air Force Center for Environmental Excellence, Technology Transfer Division, Brooks Air Force Base, San Antonio, TX
Xia Y, Cheng Y, Li L, Chen Y, Jiang Y (2020) A microcosm study on persulfate oxidation combined with enhanced bioremediation to remove dissolved BTEX in gasoline-contaminated groundwater. Biodegradation 31(3):213–222
Zhao Y, Qu D, Hou Z, Zhou R (2015) Enhanced natural attenuation of BTEX in the nitrate-reducing environment by different electron acceptors. Environ Technol 36(5):615–621
Funding
This work was supported by the National Natural Science Foundation of China (grant No. 41967028) and by the Key Project of the Guangxi Natural Science Foundation (grant No. 2019GXNSFDA 245030).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Equations 1 and 3 were presented incorrectly during the production process.
Rights and permissions
About this article
Cite this article
Wang, H., Chen, Y., He, L. et al. Feasibility of nitrate reduction combined with persulfate oxidation in the remediation of groundwater contaminated by gasoline. Hydrogeol J 30, 151–161 (2022). https://doi.org/10.1007/s10040-021-02417-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-021-02417-w