Abstract
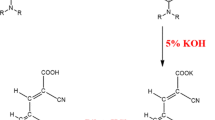
A new 7-{[1H-indol-3-ylmethylidene]amino}-4-methyl-2H-chromen-2-one dye (3) was synthesized by the reaction of 7-amino-4-methyl coumarin with indole-3-carbaldehyde in EtOH using GAA as a catalyst. The photophysical properties of the synthesized compound were studied using UV-visible and photoluminescence spectrophotometer. Redox onset potential for the dye compound was recorded through a cyclic voltammogram (CV), which aids to calculate the highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) values. The experimental and theoretical HOMO–LUMO values were calculated using the density functional theory (DFT). Moreover, the I-V characteristics were evaluated by two-sense Keithley source in dark and light medium by using a mercury lamp as a light source. The results of the I-V study showed that the compound (3) possesses good light-absorbing capability with a high molar absorption extinction coefficient (0.80 × 10−5 Ɛ). Therefore, the I-V characteristics suggest the efficiency of obtained dye for photovoltaic uses.






Similar content being viewed by others
References
Regan BO, Gratzel M (1991) A low-cost, high-efficiency solar cell based on dye sensitized colloidal TiO2 films. Nature 353(6346):737–740
Irfan A, Chaudhry AR, Al-Sehemi AG, Assiri MA, Ullah S et al (2019) Exploration of optoelectronic and photosensitization properties of triphenylamine-based organic dye on TiO2 surfaces. J Comput Electron 18(4):1119–1127
Babu AA, Shankar T, Swarnalatha K et al (2018) Co-sensitization of ruthenium(II) dye-sensitized solar cells by coumarin based dyes. J Phys Chem Lett 699:32–39
Arkan F, Izadyar M et al (2020) Optoelectronic properties and energy conversion of organic dye-based solar cells. J Opt 203:1–31
Wang ZS, Cui Y, Hara K, Dan-oh Y, Kasada C, Shinpo A et al (2007) A high light harvesting efficiency coumarin dye for stable dye-sensitized solar cells. Adv Mater 19(8):1138–1141
Mishra A, Fischer MKR, Bauerle P et al (2009) Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew Chem Int Ed 48(14):2474–2499
Seo KD, Choi IT, Park YG, Kang S, Lee JY, Kim HK et al (2012) Novel D-π-A coumarin dyes containing low band-gap chromophores for dye-sensitised solar cells. Dyes Pigments 94(3):469–474
Wang ZS, Cui Y, Dan-oh Y, Kasada C, Shinpo A, Hara K et al (2007) Thiophene-functionalized coumarin dye for efficient dye-sensitized solar cells: electron lifetime improved by coadsorption of deoxycholic acid. J Phys Chem C 111(19):7224–7230
Kuang D, Uchida S, Baker RH, Zakeeruddin SM, Grätzel Angew M et al (2008) Organic dye-sensitized ionic liquid based solar cells: remarkable enhancement in performance through molecular design of indoline sensitizers. Chem Ed 47(10):1923–1927
Xia HQ, Wang J, Bai FQ, Zhang HX et al (2015) Theoretical studies of electronic and optical properties of the triphenylamine-based organic dyes with diketopyrrolopyrrole chromophore. Dyes Pigments 113:87–95
Tanaka K, Takimiya K, Otsubo T, Kawabuchi K, Kajihara S, Harima Y et al (2006) Development and photovoltaic performance of oligothiophene-sensitized TiO2 solar cells. Chem Lett 35(6):592–593
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H et al (2010) Dye sensitized solar cells. Chem Rev 110(11):6595–6663
Alagumalai A, Fairoos MKM, Vellimalai P, Sil MC, Nithyanandhan J et al (2016) Effect of out-of-plane alkyl group’s position in dye-sensitized solar cell efficiency: a structure property relationship utilizing indoline-based unsymmetrical squaraine dyes. ACS Appl Mater Interfaces 8(51):35353–35367
Al-Amiery AA, Al-Majedy YK, Amir A, Kadhum H, Mohamad AB et al (2015) New coumarin derivative as an eco-friendly inhibitor of corrosion of mild steel in acid medium. Molecules 20(1):366–383
Manah NSA, Sulaiman L, Azman NLSM, Abidin ZHZ, Tajuddin HA, Halim NA et al (2019) Colour analysis of organic synthetic dye coating paint films consisting 4-hydroxycoumarin derivatives after exposed to UV-A. Mater Res Express 6(7):076418–076430
Venkatesh T, Bodke YD, Aditya Rao SJ et al (2020) Facile CAN catalyzed one pot synthesis of novel indol-5,8-pyrimido[4,5-d]pyrimidine derivatives and their pharmacological study. Chem Data Collect 25:100335–100347
Bhagat K, Bhagat J, Gupta MK, Singh JV, Gulati HK, Singh A, Kaur K, Kaur G, Sharma S, Rana A, Singh H, Sharma S, Bedi PMS et al (2019) Design, synthesis, antimicrobial evaluation, and molecular modeling studies of novel indolinedione-coumarin molecular hybrids. ACS Omega 4(5):8720–8730
Yan L, Li R, Shen W, Qi Z et al (2018) Multiple–color AIE coumarin–based schiff bases and potential application in yellow OLEDs. J Lumin 151:151–155
Yin OS, Ramalho JPP, Pereira AO, Martins SE, Salvador CA, Caldeira AT et al (2019) A simple method for labelling and detection of proteinaceous binders in art using fluorescent coumarin derivatives. Eur Phys J Plus 134:1–10
Li C, Wang S, Huang Y, Wen Q, Wang L, Kan Y et al (2014) Photoluminescence properties of a novel cyclometalated iridium(III) complex with coumarin-boronate and its recognition of hydrogen peroxide. Dalton Trans 43(14):5595–5602
Kadam MLM, Patil DS, Sekar N et al (2019) Red emitting coumarin based 4, 6-disubstituted-3-cyano-2-pyridones dyes synthesis, solvatochromism, linear and non-linear optical properties. J Mol Liq 276:385–398
Bisht R, Sudhakar V, Kavungathodi MFM, Karjule N, Nithyanandhan J et al (2018) Fused fluorenylindolenine-donor-based unsymmetrical squaraine dyes for dye-sensitized solar cells. ACS Appl Mater Interfaces 10(31):26335–26347
Xiang N, Gao Z, Tian G, Chen Y, Liang W, Huang J, Dong Q, Wong WY, Su J et al (2017) Novel fluorene/indole-based hole transport materials with high thermal stability for efficient OLEDs. Dyes Pigments 137:36–48
Yathisha RO, Arthoba Nayaka Y (2020) Optical and electrical properties of organic dye sensitized Cr–ZnO and Ni–CdO nanoparticles. SN Appl Sci 2(3):451–464
Hemavathi B, Jayadev V, Pradhan SC, Gokul G, Jagadisha K, Chandrashekara GK, Ramamurthy PC, Pai RK, Unni KNN, Ahipaa TN, Soman S, Balakrishna RG et al (2018) Aggregation induced light harvesting of molecularly engineered D-A-π-A carbazole dyes for dye-sensitized solar cells. Sol Energy 174:1085–1096
Basavarajappa KV, Arthoba Nayaka Y, Purushothama HT, Vinaya MM, Antony A, Poornesh P et al (2019) Optoelectronic and current-voltage studies for novel coumarin dyes. Int J Environ Anal Chem 9:1–14
Rajapaksha RD, Turner DN, Vigil J, Frolova LV, Altiga JA, Rogelj S, Ranasingh MI et al (2019) Photo-physical properties of substituted 2,3-distyryl indoles: spectroscopic, computational and biological insights. J Photochem Photobio A Chem 376:73–79
Basavarajappa KV, Arthoba Nayaka Y, Yathisha RO, Manjunatha P et al (2019) Synthesis, characterization, optical, electrochemical and current-voltage characteristics of coumarin dyes. J Fluoresc 29(5):1201–1211
Xu Y, Martin AAS (2000) The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am Mineral 85(3-4):543–556
Hou J, Guo X (2013) Active layer materials for organic solar cells, in: WCH Choy (Ed.). Springer-Verlag London 12:17–42
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR et al (2012) Avogadro an advanced semantic chemical editor, visualization, and analysis platform. Aust J Chem 4:01–33
Arunkumar A, Anbarasan PM (2019) Optoelectronic properties of a simple metal-free organic sensitizer with different spacer groups quantum chemical assessments. J Electron Mater 48(3):1522–1530
Pearson RG (1997) Maximum chemical and physical hardness Chemical hardness. J Chem Ed Chem 76(2):267–275
Mulliken RS (1934) A new electro affinity scale together with data on valence states and on valence ionization potentials and electron affinities. J Chem Phys 2(11):782–793
Basavarajappa KV, Arthoba Nayaka Y, Purushothama HT, Yathisha RO, Vinay MM, Rudresha BJ, Manjunatha KB et al (2020) Optical, electrochemical and current-voltage characteristics of novel coumarin based 2,4-dinitrophenylhydrazone derivatives. J Mol Struct 1199:126946–126957
Stanley A, Matthews D (1995) The dark current at the TiO2 electrode of a dye-sensitized TiO2 photovoltaic cell. Aust J Chem 48(7):1293–1300
Tai Q, Yan F (2013) In: Choy WCH (ed) Organic solar cells, green energy and technology. Springer, London, pp 243–265
Acknowledgments
The authors express gratefulness to the Chairman, Department of PG studies and research in chemistry, Kuvempu University, and are thankful to IISc Bangalore for providing the spectral data.
Funding
The authors are thankful to the UGC, New Delhi, UGC-BSR start-up grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 228 kb)
Rights and permissions
About this article
Cite this article
Venkatesh, T., Upendranath, K. & Nayaka, Y.A. Development of electrochemical and optoelectronic performance of new 7-{[1H-indol-3-ylmethylidene]amino}-4-methyl-2H-chromen-2-one dye. J Solid State Electrochem 25, 1237–1244 (2021). https://doi.org/10.1007/s10008-020-04892-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04892-9