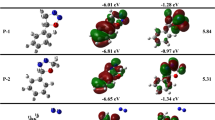
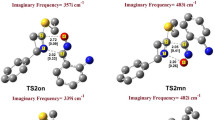
Abstract
The mechanism and regioselectivity of [3+2] cycloaddition (32CA) reactions of benzonitrile oxide with ethyl trans-cinnamate, ethyl crotonate and trans-2-penten-1-ol has been studied in gas phase and in acetonitrile, ethyl acetate and tetrahydrofuran using the B3LYP functional in connection with 6-31G(d) basis set. The 32CA reactions followed one-step mechanism with asynchronous TSs. The calculated global electron density transfer (GEDT) at the TSs showed electronic flux from benzonitrile oxide to ethyl trans-cinnamate and ethyl crotonate, while the electronic flux from trans-2-penten-1-ol to benzonitrile oxide was predicted, in complete agreement with the Conceptual Density Functional Theory (CDFT) indices. The regioselectivity is correctly described in coherence with the experiment data. The intermolecular interaction at the TSs was realized through visualization and quantification by means of independent gradient model (IGM) analysis based on promolecular density.






Similar content being viewed by others
References
Toldo JM, Merlo AA, Gonçalves PFB (2016) The 1,3-dipolar cycloaddition of nitrile oxide to vinylacetic acid: computational study of transition states selectivity, solvent effects, and bicyclo formation. J Braz Chem Soc 27:1202–1216. https://doi.org/10.5935/0103-5053.20160016
Yoshimura A, Middleton KR, Todora AD, Kastern BJ, Koski SR, Maskaev AV, Zhdankin VV (2013) Hypervalent iodine catalyzed generation of nitrile oxides from oximes and their cycloaddition with alkenes or alkynes. Org Lett 15:4010–4013. https://doi.org/10.1021/ol401815n
Ajay Kumar K, Govindaraju M, Jayaroopa P, Vasanth Kumar G (2013) Nitrile oxides: a key intermediate in organic synthesis. Int J Pharm Chem Biol Sci 3:91–101
Cheng J-F, Mjalli AMM (1998) Solid-phase synthesis of Δ2-isoxazolines. Tetrahedron Lett 39:939–942. https://doi.org/10.1016/S0040-4039(97)10700-6
Wityak J, Sielecki TM, Pinto DJ, Emmett G, Sze JY, Liu J, Tobin E, Shuaige W, Jiang B, Ma P, Mousa SA, Wexler RR, Olson RE (1997) Discovery of potent Isoxazoline glycoprotein IIb/IIIa receptor antagonists. J Med Chem 40:50–60. https://doi.org/10.1021/jm960626t
Confalone PN, Lollar ED, Pizzolato G, Uskokovic MR (1978) Use of intramolecular [3 + 2] cycloaddition reactions in the synthesis of natural products. A stereospecific synthesis of (.+-.)-biotin from cycloheptene. J Am Chem Soc 100:6291–6292. https://doi.org/10.1021/ja00487a086
Padwa A (1984) 1,3-dipolar cycloaddition chemistry. Wiley, New York
Huisgen R (1968) Mechanism of 1,3-dipolar cycloadditions. Reply J Org Chem 33:2291–2297. https://doi.org/10.1021/jo01270a024
Huisgen R (1976) 1,3-Dipolar cycloadditions. 76. Concerted nature of 1,3-dipolar cycloadditions and the question of diradical intermediates. J Org Chem 41:403–419. https://doi.org/10.1021/jo00865a001
Grundmann C, Grünanger P (1971) The nitrile oxides: versatile tools of theoretical and preparative chemistry. Springer-Verlag, Berlin Heidelberg
Firestone RA (1968) Mechanism of 1,3-dipolar cycloadditions. J Org Chem 33:2285–2290. https://doi.org/10.1021/jo01270a023
Vullo V, Danks TN, Wagner G (2004) Cycloaddition of benzonitrile oxide to acetonitrile, propyne and propene − a comparative theoretical study of the reaction mechanism and regioselectivity. Eur J Org Chem 2004:2046–2052. https://doi.org/10.1002/ejoc.200400010
Fukui K, Yonezawa T, Shingu H (1952) A molecular orbital theory of reactivity in aromatic hydrocarbons. J Chem Phys 20:722–725. https://doi.org/10.1063/1.1700523
Domingo LR, Chamorro E, Pérez P (2009) An analysis of the regioselectivity of 1,3-dipolar cycloaddition reactions of benzonitrile N-oxides based on global and local electrophilicity and nucleophilicity indices. Eur J Org Chem 2009:3036–3044. https://doi.org/10.1002/ejoc.200900213
Jeong J, Zong K, Choe JC (2017) Regioselectivity of 1,3-dipolar cycloadditions of benzonitrile oxide to alkenyl boronic esters: an experimental and computational study. J Het Chem 54:1007–1014. https://doi.org/10.1002/jhet.2667
Jasiński R, Jasińska E, Dresler E (2017) A DFT computational study of the molecular mechanism of [3 + 2] cycloaddition reactions between nitroethene and benzonitrile N-oxides. J Mol Model 23:13. https://doi.org/10.1007/s00894-016-3185-8
Miroslaw B, Babyuk D, Lapczuk-Krygier A, Kacka-Zych A, Dumchuk OM, Jasiński R (2018) Regiospecific formation of the nitromethyl-substituted 3-phenyl-4,5-dihydroisoxazole via [3 + 2] cycloaddition. Monatsh Chem 149:1877–1884. https://doi.org/10.1007/s00706-018-2227-6
Woliński P, Kacka-Zych A, Dumchuk OM, Lapczuk-Krygier A, Miroslaw B, Jasiński R (2020) Clean and molecularly programmable protocol for preparation of bis heterobiarylic systems via a domino pseudocyclic reaction as a valuable alternative for TM-catalyzed cross-couplings. J Clean Prod 275:122086. https://doi.org/10.1016/j.jclepro.2020.122086
Acharjee N (2020) Theoretical analysis of the regio- and stereoselective synthesis of spiroisoxazolines. J Mol Model 26:117. https://doi.org/10.1007/s00894-020-04372-x
Lapczuk-Krygier A, Jaśkowska J, Jasiński R (2018) The influence of Lewis acid catalyst on the kinetic and molecular mechanism of nitrous acid elimination from 5-nitro-3-phenyl-4,5-dihydroisoxazole: DFT computational study. Chem Heterocycl Compd 54:1172–1174. https://doi.org/10.1007/s10593-019-02410-y
Jasiński R (2015) Nitroacetylene as dipolarophile in [2 + 3] cycloaddition reactions with allenyl-type three-atom components: DFT computational study. Monatsh Chem 146:591–599. https://doi.org/10.1007/s00706-014-1389-0
Rosella CE, Harper JB (2009) The effect of ionic liquids on the outcome of nitrile oxide cycloadditions. Tetrahedron Lett 50:992–994. https://doi.org/10.1016/j.tetlet.2008.12.045
Dresler E, Kacka-Zych A, Kwiatkowska JR (2018) Regioselectivity, stereoselectivity, and molecular mechanism of [3 + 2] cycloaddition reactions between 2-methyl-1-nitroprop-1-ene and (Z)-C-aryl-N-phenylnitrones: a DFT computational study. J Mol Model 24:329. https://doi.org/10.1007/s00894-018-3861-y
Domingo LR, Acharjee N (2020) A molecular electron density theory study of the Grignard reagent-mediated regioselective direct synthesis of 1,5-disubstituted-1,2,3-triazoles. J Phys Org Chem 33:e4062. https://doi.org/10.1002/poc.4062
Alnajjar RA, Jasiński R (2019) Competition between [2 + 1]- and [4 + 1]-cycloaddition mechanisms in reactions of conjugated nitroalkenes with dichlorocarbene in the light of a DFT computational study. J Mol Model 25:157. https://doi.org/10.1007/s00894-019-4006-7
Adjieufack AI, Ndassa IM, Mbadcam JK, Gutiérrez MR, Domingo LR (2017) Understanding the reaction mechanism of the Lewis acid (MgBr2)-catalysed[3+2] cycloaddition reaction between C-methoxycarbonyl nitrone and 2-propen-1-ol: a DFT study. Theor Chem Accounts 136:5. https://doi.org/10.1007/s00214-016-2028-0
Geerlings P, de Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1873. https://doi.org/10.1021/cr990029p
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21:748. https://doi.org/10.3390/molecules21060748
Domingo LR (2014) A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv 4:32415–32418. https://doi.org/10.1039/C4RA04280H
Lefebvre C, Khartabil H, Boisson J-C, Contreras-García J, Piquemal J-P, Hénon E (2018) The independent gradient model: a new approach for probing strong and weak interactions in molecules from wave function calculations. ChemPhysChem 19:724–735. https://doi.org/10.1002/cphc.201701325
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules, vol 4. Oxford University Press, Oxford, pp 70–86
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Hehre WJ, Radom L, Schleyer P v R, Pople J (1986) AB INITIO molecular orbital theory1st edn. Wiley-Interscience, New York
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 58:4417–4423. https://doi.org/10.1016/S0040-4020(02)00410-6
Parr RG, Szentpály L v, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Domingo LR, Pérez P (2011) The nucleophilicity N index in organic chemistry. Org. Biomol. Chem. 9: 7168–7175. doi: https://doi.org/10.1039/C1OB05856H
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746. https://doi.org/10.1063/1.449486
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926. https://doi.org/10.1021/cr00088a005
Fukui K (1970) Formulation of the reaction coordinate. J Phys Chem 74:4161–4163. https://doi.org/10.1021/j100717a029
González C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527. https://doi.org/10.1021/j100377a021
González C, Schlegel HB (1991) Improved algorithms for reaction path following: higher-order implicit algorithms. Chem Phys 95:5853–5860. https://doi.org/10.1063/1.461606
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094. https://doi.org/10.1021/cr00031a013
Simkin BI (1995) Quantum chemical and statistical theory of solutions : a computational approach. Ellis Horwood, London
Cossi M, Barone V, Cammi R, Tomasi J (1996) Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett 255:327–335. https://doi.org/10.1016/0009-2614(96)00349-1
Cancès E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041. https://doi.org/10.1063/1.474659
Barone V, Cossi M, Tomasi J (1998) Geometry optimization of molecular structures in solution by the polarizable continuum model. J Comput Chem 19:404–417. https://doi.org/10.1002/(SICI)1096-987X(199803)19:4<404::AID-JCC3>3.0.CO;2-W
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136:B864–B871. https://doi.org/10.1103/PhysRev.136.B864
Corminboeuf C (2014) Minimizing density functional failures for non-covalent interactions beyond van der Waals complexes. Acc Chem Res 47:3217–3224. https://doi.org/10.1021/ar400303a
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Gaussian 09, Revision A.02,Gaussian, Inc., Wallingford CT, 2009
Ríos-Gutiérrez M, Domingo LR (2019). Eur J Org Chem:267–282. https://doi.org/10.1002/ejoc.201800916
Wiberg KB (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24:1083–1096. https://doi.org/10.1016/0040-4020(68)88057-3
Lendvay G (1994) Characterization of the progress of chemical reactions by ab initio bond orders. J Phys Chem 98:6098–6104. https://doi.org/10.1021/j100075a009
García JI, Martínez-Merino V, Mayoral JA, Salvatella L (1998) Density functional theory study of a Lewis acid catalyzed Diels−Alder reaction. The butadiene + acrolein paradigm. J Am Chem Soc 120:2415–2420. https://doi.org/10.1021/ja9722279
Froese RDJ, Organ MG, Goddard JD, Stack TDP, Trost BM (1995) Theoretical and experimental studies of the Diels-Alder dimerizations of substituted cyclopentadienes. J Am Chem Soc 117:10931–10938. https://doi.org/10.1021/ja00149a016
Bach RD, McDouall JJW, Schlegel HB, Wolber GJ (1989) Electronic factors influencing the activation barrier of the Diels-Alder reaction. An ab initio study. J Org Chem 54:2931–2935. https://doi.org/10.1021/jo00273a029
Marakchi K, Kabbaj OK, Komiha N (2002) Etude DFT du mécanisme des réactions de cycloaddition dipolaire-1,3 de la C,N-diphénylnitrone avec des dipolarophiles fluorés de type éthylénique et acétylénique. J Fluor Chem 114:81–89. https://doi.org/10.1016/S0022-1139(01)00570-X
Domingo LR, Ríos-Gutiérrez M, Emamian S (2016) Understanding the stereoselectivity in Brønsted acid catalysed Povarov reactions generating cis/trans CF3-substituted tetrahydroquinolines: a DFT study. RSC Adv 6:17064–17073. https://doi.org/10.1039/C5RA27650K
Nacereddine AK, Sobhi C, Djerourou A, Ríos-Gutiérrez M, Domingo LR (2015) Non-classical CH⋯O hydrogen-bond determining the regio- and stereoselectivity in the [3 + 2] cycloaddition reaction of (Z)-C-phenyl-N-methylnitrone with dimethyl 2-benzylidenecyclopropane-1,1-dicarboxylate. A topological electron-density study. RSC Adv 5:99299–99311. https://doi.org/10.1039/C5RA20268J
Lefebvre C, Rubez G, Khartabil H, Boisson J-C, Contreras-García J, Hénon E (2017) Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys Chem Chem Phys 19:17928–17936. https://doi.org/10.1039/C7CP02110K
Salah M, Zeroual A, Jorio S, El Hadki H, Kabbaj OK, Marakchi K, Komiha N (2020) Theoretical study of the 1,3-DC reaction between fluorinated alkynes and azides: reactivity indices, transition structures, IGM and ELF analysis. J Mol Graphics Model 94:107458. https://doi.org/10.1016/j.jmgm.2019.107458
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) An MEDT study of the carbenoid-type [3 + 2] cycloaddition reactions of nitrile ylides with electron-deficient chiral oxazolidinones. Org Biomol Chem 14:10427–10436. https://doi.org/10.1039/C6OB01989G
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Tables with total energies, enthalpies, free energies and entropies and tables with calculated bond lengths and bond angles of the stationary points optimized along the 32CA reaction of BNO with 1b, 2b and 3b. (DOCX 3482 kb)
Rights and permissions
About this article
Cite this article
Abbiche, K., Acharjee, N., Salah, M. et al. Unveiling the mechanism and selectivity of [3+2] cycloaddition reactions of benzonitrile oxide to ethyl trans-cinnamate, ethyl crotonate and trans-2-penten-1-ol through DFT analysis. J Mol Model 26, 279 (2020). https://doi.org/10.1007/s00894-020-04547-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04547-6