Abstract
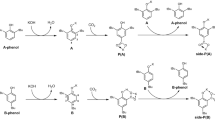
The microcosmic mechanism of the Menshutkin reaction between DABCO and benzyl fluoride/fluorodiphenylmethane has been investigated in both the gas and solvent phase by performing DFT calculations at B3LYP/6-31G(d,p) level of theory. The Gibbs free energy profiles to reach the possible transition states, i.e., five-membered ring transition state and SN2 transition state show that the reaction between DABCO and benzyl fluoride proceeds through SN2 transition state in accordance with previously reported studies, while the reaction between DABCO and fluorodiphenylmethane proceeds through five-membered ring transition state contrary to earlier literature. The role of solvent has been elucidated by reoptimizing the structures using SMD model of solvation. Hydrogen bonding and steric hinderance have been identified as the key factors in guiding the reaction pathway of commercially important Menshutkin reaction.








Similar content being viewed by others
References
Welton T (1999) Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev 99:2071–2083
Brennecke JF, EJ (2001) Ionic liquids: innovation of fluids for chemical processing 47:2384
Zhang SJ, Lv XM (2006) Ionic liquids-from fundamentals to applications. Scientific, Beijing, China
Huddleston JG, Visser AE, Reichert WM, Willauer HD, Brooker GA, Rogers RD (2001) Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating imidazolium cation. Green Chem 3:156–164
Wassercheld P, Keim W (2000) Ionic liquids-new “solutions” for transition metal catalysis. Angew Chem Int Ed 39:3772–3789
Hagiwara R, Ito Y (2000) The room temperature ionic liquids of alkylimidazolium cations and fluoroanioins. J Fluor Chem 105:221–227
Earle MJ, Seddon KR (2000) Ionic liquids. Green solvents for future. Pure Appl Chem 72:1391–1398
Gu YL, Shi F, Deng YQ (2004) Esterification of aliphatic acids with olefin promoted by Bronsted acidic ionic liquids. J Mol Catal A Chem 212:71–75
Xing HB, Wang T, Zhou ZH, Dai YY (2007) The sulfonic-acid functionalized ionic liquids with pyridinium cations: acidities and their acidity-catalytic activity relationship. J Mol Catal A Chem 264:53–59
Seddon KR (1997) Ionic liquids for clean technology. Chem Technol Biotechnol 68:351–356
Sheldon R (2001) Catalytic reactions in ionic liquids. Chem Commun 23:2399–2407
Carmicheal AJ, Earle MJ, Holbery JD, McCormac PB, Seddon KR (1999) The Heck reaction in ionic liquids: a multiphasic catalytic system. Org Lett 1:997–1000
Bates ED, Mayton RD, Ntai I, Davis JH (2002) CO2 capture by a task-specific ionic liquid. J Am Chem Soc 124:926–927
Mjewski P, Pernak A, Grzymislawski M, Iwanik K, Pernak J (2003) Ionic liquid in embalming and tissue preservation: can traditional formalin-fixation be replaced safely? Acta Histochem 105:35–39
Chun S, Dzyuba SV, Bartsch RA (2001) Influence of structural variation in room-temperature ionic liquids on the selectivity and efficiency of competitive alkali metal salt extraction by crown ether. Anal Chem 73:3737–3741
Fadeev AG, Meagher MM (2001) Opportunities for ionic liquids in recovery of biofules. Chem Commun 3:295–296
Chen CY, Burton AW Jr, Liang AJ (2006) Molecular sieves SSZ-71 composition of matter and synthesis thereof. US Patent US7083776 B2
Acevedo O, Jorgensen WL (2010) Exploring solvents effect upon Menshutkin reaction using a polarisable force field. J Phys Chem B 114:8425–8430
Andre M, Antonio JIA, Joao CRR, Antonio RTC (2006) Unusual solvent effects on a SN2 Reaction: a quantum-mechanical and kinetic study of the menshutkin reaction between 2-amino-1-methyl-benzimidazole and iodomethane in gas phase and acetonitrile. J Phys Chem B 110:1877–1888
Zhu X, Zhang D, Liu C (2011) New insight into the formation mechanism of imidazolium-based halide salts. J Mol Model 17:2099–2102
Zhu X, Cui P, Zhang D, Liu C (2011) Theoretical study for pyridinium-based ionic liquid 1-ethylpyridinium trifluoroacetate: synthesis, electronic structure, and catalytic reactivity. J Phys Chem A 115:8255–8263
Wang Y, Li HR, Wu T, Wang CM, Han SJ (2005) Reaction mechanism study for the synthesis of alkylimidazolium-based halide ionic liquids. Acta Phys -Chim Sin 21:517–522
Singh A, Singh P, Goel N (2013) Theoretical study of DABCO-based ionic liquid: synthesis and reaction mechanism. Struct Chem. doi:10.1007/s11224-013-0348-4
Becke AD (1993) Density-functionalcthermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Clark T, Chandrashekhar J, Spitzangel GW, Schelyer PVR (1983) Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21 + G basis set for first row elements, Li-F. J Comput Chem 4:294–301
Francl MM, Pietro WJ, Herhe WJ, Binkley JS, Defrees DJ, Pople JA, Gordon MS (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second row elements. J Chem Phys 77:3654–3655
Gordon MS (1980) The isomers of silacyclopropane. Chem Phys Lett 76:163–168
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE et al. (2010) Gaussian 09, Revision C01. Gaussian Inc, Wallingford
Fukui K (1981) The path of chemical reactions-the irc approach. Acc Chem Res 14:363–368
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Wei S, Yalalov DA, Tsogoeva SB, Schmatz S (2007) New highly enantioselective thiourea-based bifunctional organocatalysts for nitro-Michael addition reactions. Catal Today 121:151–157
Acknowledgments
A.P.S. thanks the Council of Scientific and Industrial Research, India (No. 09/135(0650)/2011-EMR-I) for the senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A., Goel, N. Menshutkin reaction between DABCO and benzyfluoride/fluorodiphenylmethane: a mechanistic study. J Mol Model 20, 2265 (2014). https://doi.org/10.1007/s00894-014-2265-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2265-x