Abstract
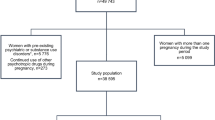
The prevention of relapses and the treatment of depression during pregnancy are difficult challenges. The maintenance of antidepressants in pregnancy with its concomitant risks to mother and child needs to be weighed against those associated with not treating the disease. This study aimed at quantifying the impact of the occurrence of pregnancy on the course of antidepressant treatment among newly treated women (< 6 months). We performed a comparative observational cohort study using the nationwide French reimbursement healthcare system database. Women who conceived in 2014 and initiated an antidepressant at any time in the 6 months before pregnancy were compared with nonpregnant women newly exposed to antidepressants with matching on age, antidepressant exposure, history of psychiatric disorders, and area of residence. The primary outcome was a composite of antidepressant discontinuation, switch to another antidepressant, and concomitant use of antidepressants. The secondary outcome was the resumption of antidepressant during follow-up. We used Cox marginal proportional hazards models to compare time to outcomes between pregnant and nonpregnant women. The pregnant cohort included 6593 women, and the comparison cohort 29,347 nonpregnant women. In the period following the first month of treatment, pregnant women were more likely to experience treatment modification, and especially to stop receiving it, compared with nonpregnant women (adjusted hazard ratio (aHR) 1.58; 95%CI, 1.51–1.62). Pregnant women who discontinued treatment had a 41% decreased incidence of antidepressant resumption compared with nonpregnant women (aHR 0.59; 95%CI, 0.56–0.62). Pregnancy was a determinant of antidepressant treatment modification, and especially of discontinuation.




Similar content being viewed by others
References
Benard-Laribiere A, Pambrun E, Sutter-Dallay AL, Gautier S, Hurault-Delarue C, Damase-Michel C, Lacroix I, Begaud B, Pariente A (2018) Patterns of antidepressant use during pregnancy: a nationwide population-based cohort study. Br J Clin Pharmacol 84:1764–1775
Berard A, Sheehy O (2014) The Quebec Pregnancy Cohort--prevalence of medication use during gestation and pregnancy outcomes. PLoS One 9:e93870
Berard A, Sheehy O, Zhao JP, Chambers C, Roth M, Bozzo P, Johnson D, Kao K, Lavigne S, Wolfe L, Quinn D, Dieter K, MotherToBaby Collaborative Research C (2019) Impact of antidepressant use, discontinuation, and dosage modification on maternal depression during pregnancy. Eur Neuropsychopharmacol 29:803–812
Charlton RA, Jordan S, Pierini A, Garne E, Neville AJ, Hansen AV, Gini R, Thayer D, Tingay K, Puccini A, Bos HJ, Nybo Andersen AM, Sinclair M, Dolk H, de Jong-van den Berg LT (2015) Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population-based study in six European regions. BJOG 122:1010–1020
Cooper WO, Willy ME, Pont SJ, Ray WA (2007) Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol 196(544):e541–e545
Gentile S (2017) Untreated depression during pregnancy: short- and long-term effects in offspring. A systematic review. Neuroscience 342:154–166
Howard LM, Megnin-Viggars O, Symington I, Pilling S, Guideline Development G (2014) Antenatal and postnatal mental health: summary of updated NICE guidance. BMJ 349:g7394
Hurault-Delarue C, Lacroix I, Benard-Laribiere A, Montastruc JL, Pariente A, Damase-Michel C (2019) Antidepressants during pregnancy: a French drug utilisation study in EFEMERIS cohort. Eur Arch Psychiatry Clin Neurosci 269:841–849
Institut National d'Etudes Démographiques, 2012. Statistiques d'IVG. http://ivg_statistiques.site.ined.fr. Accessed 25 April 2019
Leong C, Raymond C, Chateau D, Dahl M, Alessi-Severini S, Falk J, Bugden S, Katz A (2017) Psychotropic drug use before, during, and after pregnancy: a population-based study in a Canadian cohort (2001-2013). Can J Psychiatr 62:543–550
Margulis AV, Kang EM, Hammad TA (2014) Patterns of prescription of antidepressants and antipsychotics across and within pregnancies in a population-based UK cohort. Matern Child Health J 18:1742–1752
Margulis AV, Palmsten K, Andrade SE, Charlton RA, Hardy JR, Cooper WO, Hernandez-Diaz S (2015) Beginning and duration of pregnancy in automated health care databases: review of estimation methods and validation results. Pharmacoepidemiol Drug Saf 24:335–342
Molyneaux E, Telesia LA, Henshaw C, Boath E, Bradley E, Howard LM (2018) Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev 4:CD004363
National Institute for Health and Care Excellence, 2014. Antenatal and postnatal mental health: clinical management and service guidance (update). (Clinical Guideline 192.). http://guidance.nice.org.uk/CG192. Accessed 15 July 2019
National Institute for Health and Care Excellence, 2018. Antenatal and postnatal mental health: the NICE guideline on clinical management and service guidance (updated edition). (National Clinical Guidance Number 192). https://www.nice.org.uk/guidance/cg192/evidence/full-guideline-pdf-4840896925. Accessed 15 July 2019
Olfson M, Marcus SC, Tedeschi M, Wan GJ (2006) Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry 163:101–108
Petersen I, Gilbert RE, Evans SJ, Man SL, Nazareth I (2011) Pregnancy as a major determinant for discontinuation of antidepressants: an analysis of data from The Health Improvement Network. J Clin Psychiatry 72:979–985
Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158:915–920
Tuppin P, Rudant J, Constantinou P, Gastaldi-Menager C, Rachas A, de Roquefeuil L, Maura G, Caillol H, Tajahmady A, Coste J, Gissot C, Weill A, Fagot-Campagna A (2017) Value of a national administrative database to guide public decisions: from the systeme national d'information interregimes de l'Assurance Maladie (SNIIRAM) to the systeme national des donnees de sante (SNDS) in France. Rev Epidemiol Sante Publique 65(Suppl 4):S149–S167
Verdoux H, Cougnard A, Thiebaut A, Tournier M (2011) Impact of duration of antidepressant treatment on the risk of occurrence of a new sequence of antidepressant treatment. Pharmacopsychiatry 44:96–101
Zoega H, Kieler H, Norgaard M, Furu K, Valdimarsdottir U, Brandt L, Haglund B (2015) Use of SSRI and SNRI antidepressants during pregnancy: a population-based study from Denmark, Iceland, Norway and Sweden. PLoS One 10:e0144474
Funding
This work was supported by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, ANSM) (grant number 2014S029). The present study is part of the Drugs Systematized Assessment in real-liFe Environment (DRUGS-SAFE) research program. This program aims at providing an integrated system allowing the concomitant monitoring of drug use and safety in France. The potential impact of drugs, frailty of populations, and seriousness of risks drive the research program. This publication represents the views of the authors and does not necessarily represent the opinion of the French Medicines Agency.
Author information
Authors and Affiliations
Contributions
ABL, ALSD, SG, CHD, CDM, IL, and AP conceptualized and designed the work.
EP collected the data and carried out the analysis.
ABL, ALSD, SG, CHD, CDM, IL, and AP interpreted the data.
ABL wrote the first draft, and all the authors critically revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Neither ethics committee approval nor informed consent was required for this observational study based on French medico-administrative databases because of the anonymous nature of the data, by agreement of the French Data Protection Supervisory Authority (Commission nationale de l’informatique et des libertés).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bénard-Laribière, A., Pambrun, E., Sutter-Dallay, AL. et al. Impact of pregnancy on antidepressant treatment course: a population-based comparative cohort study in France. Arch Womens Ment Health 23, 699–707 (2020). https://doi.org/10.1007/s00737-020-01033-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00737-020-01033-z