Abstract
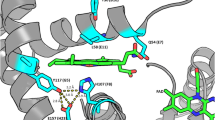
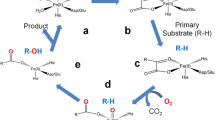
Three distinct electron paramagnetic resonance (EPR) spectra of radical intermediates formed as reactive intermediates in the catalytic cycle of Synechocystis catalase–peroxidase were identified. Multifrequency EPR spectroscopy, combined with site-directed mutagenesis and selective deuterium labeling of Trp and Tyr residues, allowed us to unequivocally assign such intermediates to an [Fe(IV) = O Por·+] species, the first committed intermediate in monofunctional peroxidases and two protein-based radicals, identified as Trp106· and a Tyr·, formed subsequently to the [Fe(IV) = O Por·+] species by intramolecular electron transfer. Our recent characterization of the Mycobacterium tuberculosis catalase–peroxidase showed that the Trp· sites differ among these enzymes, and that the [Fe(IV) = O Trp·] species was the reactive intermediate with the prodrug isoniazid. Accordingly, the question to address was whether the dissimilarity in the sites for the formation of the Trp· intermediates and in the geometry of the distal side was reflected by differences in the peroxidase-like reaction of Synechocystis and Mycobacterium tuberculosis catalase–peroxidases with the prodrug isoniazid. Our findings show that in the Synechocystis enzyme, the isoniazid substrate can get closer to the heme distal side and can react readily with the [Fe(IV) = O Por·+] species, at variance to the situation in the M. tuberculosis catalase–peroxidase. These results indicate that, as in the case of monofunctional peroxidases, the difference in the sites for the formation of the Trp· as alternative reactive intermediates to the [Fe(IV) = O Por·+] species is correlated to differences in substrate binding sites.




Similar content being viewed by others
References
K.G. Welinder, Biochim. Biophys. Acta 1080, 215–220 (1991)
M.G. Klotz, P.C. Loewen, Mol. Biol. Evol. 20, 1098–1112 (2003)
F. Passardi, M. Zamocky, J. Favet, C. Jakopitsch, C. Penel, C. Obinger, C. Dunand, Gene 397, 101–113 (2007)
Y. Zhang, B. Heym, B. Allen, D. Young, S. Cole, Nature 358, 591–593 (1992)
T. Bertrand, N.A. Eady, J.N. Jones, J.M. Nagy, B. Jamart-Gregoire, E.L. Raven, K.A. Brown, J. Biol. Chem. 279, 38991–38999 (2004)
G. Smulevich, C. Jakopitsch, E. Droghetti, C. Obinger, J. Inorg. Biochem. 100, 568–585 (2006)
R. Singh, J. Switala, P.C. Loewen, A. Ivancich, J. Am. Chem. Soc. 129, 15954–15963 (2007)
M. Sivaraja, D.B. Goodin, M. Smith, B.M. Hoffman, Science 245, 738–740 (1989)
A. Ivancich, P. Dorlet, D.B. Goodin, S. Un, J. Am. Chem. Soc. 123, 5050–5058 (2001)
C. Jakopitsch, F. Ruker, G. Regelsberger, M. Dockal, G.A. Peschek, C. Obinger, Biol. Chem. 380, 1087–1096 (1999)
C. Jakopitsch, J. Vlasits, B. Wiseman, P.C. Loewen, C. Obinger, Biochemistry 46, 1183–1193 (2007)
A. Ivancich, C. Jakopitsch, M. Auer, S. Un, C. Obinger, J. Am. Chem. Soc. 125, 14093–14102 (2003)
C. Jakopitsch, C. Obinger, S. Un, A. Ivancich, J. Inorg. Biochem. 100, 1091–1099 (2006)
S. Un, P. Dorlet, A.W. Rutherford, Appl. Magn. Reson. 21, 341–361 (2001)
S. Un, Magn. Reson. Chem. 43, S229–S239 (2005)
M. Perez-Boada, F.J. Ruiz-Dueñas, R. Pogni, R. Basosi, T. Choinowski, M.J. Martínez, K. Piontek, A.T. Martínez, J. Mol. Biol. 354, 385–402 (2005)
B.H. Dunford, Heme Peroxidases (Wiley, New York, 1999)
C. Jakopitsch, E. Droghetti, F. Schmuckenschlager, P.G. Furtmüller, G. Smulevich, C. Obinger, J. Biol. Chem. 280, 42411–42422 (2005)
C. Jakopitsch, M. Auer, G. Regelsberger, W. Jantschko, P.G. Furtmuller, F. Ruker, C. Obinger, Biochemistry 42, 5292–5300 (2003)
R. Pierattelli, L. Banci, N.A. Eady, J. Bodiguel, J.N. Jones, P.C. Moody, E.L. Raven, B. Jamart-Gregoire, K.A. Brown, J. Biol. Chem. 279, 39000–39009 (2004)
C. Metcalfe, I.K. Macdonald, E.J. Murphy, K.A. Brown, E.L. Raven, P.C.E. Moody, J. Biol. Chem. 283, 6193–6200 (2008)
C. Jakopitsch, A. Ivancich, F. Schmuckenschlager, A. Wanasinghe, G. Potl, P.G. Furtmuller, F. Ruker, C. Obinger, J. Biol. Chem. 279, 46082–46095 (2004)
W.A. Doyle, W. Blodig, N.C. Veitch, K. Piontek, A.T. Smith, Biochemistry 37, 15097–15105 (1998)
W. Blodig, A.T. Smith, W.A. Doyle, K. Piontek, J. Mol. Biol. 305, 851–861 (2001)
J. Colin, B. Wiseman, J. Switala, P.C. Loewen, A. Ivancich, J. Am. Chem. Soc. 131, 8557–8563 (2009)
Acknowledgments
This work was supported by the French Centre National de la Recherche Scientifique and Commissariat à l'Énergie Atomique (CEA, Saclay, to A.I.), and PhD. Fellowship (CFR contract from CEA Saclay to J.C.) and by the Austrian Science Fund, Project (FWF P18751 to C.O.). We thank Sun Un (CEA Saclay) for insights and discussions on HF-EPR spectroscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colin, J., Jakopitsch, C., Obinger, C. et al. The Reaction of Synechocystis Catalase–Peroxidase (KatG) with Isoniazid Investigated by Multifrequency (9–285 GHz) EPR Spectroscopy. Appl Magn Reson 37, 267–277 (2010). https://doi.org/10.1007/s00723-009-0080-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-009-0080-9