Abstract
The staining pattern of 1,2-bis(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-undecanoyl)-sn-glycero-3-phosphocholine (Bodipy PC) was investigated in internodal cells of the green alga Chara corallina. Ten minutes after dye addition, Bodipy-PC-derived fluorescence appeared in lipid droplets and after 1 h in the cortical endoplasmic reticulum (ER) and in the inner ER tubes. Staining of the ER required energy but was independent of an intact actin or microtubule cytoskeleton and independent of vesicular endocytosis. The size of the lipid droplets varied between 0.25 µm in elongating cells and 3.2 µm in senescent internodes. They moved together with or along the cortical ER cisternae in a cytoskeleton-independent manner or remained immobile up to several minutes. Detachment of lipid droplets from the cortical ER or fusion of lipid droplets was never observed. The results of this study suggest that Bodipy PC is a valuable, less toxic alternative to 3,3′-dihexyloxacarbocyanine iodide (DiOC6) staining of the ER in Chara. They confirm an earlier report about microtubule-dependent cortical ER morphology and dynamics in elongating internodes and offer new perspectives for the study of organelle interactions.





Similar content being viewed by others
Abbreviations
- Bodipy PC:
-
Bis-BODIPY© FL C11-PC = 1,2-bis-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-undecanoyl)-sn-glycero-3-phosphocholine
- CD:
-
Cytochalasin D
- CLSM:
-
Confocal laser scanning microscope/microscopy
- DIC:
-
Differential interference contrast
- DiOC6 :
-
3,3′-Dihexyloxacarbocyanine iodide
- DMSO:
-
Dimethyl sulfoxide
- DNP:
-
2,4-Dinitrophenole
- EDTA:
-
Ethylenediaminetetraacetic acid
- EGTA:
-
Ethylene glycol tetraacetic acid
- IPC:
-
Isopropyl N-(3-chlorophenyl)-carbamate
- fps:
-
Frames per second
- NBD PC:
-
1-Acyl-2-(N-4-nitrobenz∼2-oxa-l,3-diazole)aminoacyl phosphatidyl choline
- PIPES:
-
Piperazine-N,N′-bis (2-ethanesulfonic acid)
References
Betz WJ, Mao F, Smith CB (1996) Imaging exocytosis and endocytosis. Curr Opin Neurobiol 6:365–371
Bostrom P, Rutberg M, Ericsson J, Holmdahl P, Andersson L, Frohman MA, Boren J, Olofsson S-O (2005) Cytosolic lipid droplets increase in size by microtubule-dependent complex formation. Arterioscler Thromb Vasc Biol 25:1945–1951
Cajall Y, Rogers JC, Berg OG, Jain MK (1996) Intermembrane molecular contacts by polymyxin B mediate exchange of phospholipids. Biochemistry 35:229–308
Chandra P, Heinstein PF, Low PS (1996) Activation of phospholipase A by plant defense elicitors. Plant Physiol 110:979–986
Craig S, Staehelin L (1988) High pressure freezing of intact plant tissues. Evaluation and characterization of novel features of the endoplasmic reticulum and associated membrane systems. Eur J Cell Biol 46:80–93
Dubrovsky JG, Guttenberger M, Saralegui A, Napsucialy-Mendivil S, Voigt B, Baluska F, Menzel D (2006) Neutral red as a probe for confocal laser scanning microscopy studies of plant roots. Ann Bot 97:1127–1138
Farber SA, Olson ES, Clark JD, Halpern ME (1999) Characterization of Ca2-dependent phospholipase A2 activity during zebrafish embryogenesis. J Biol Chem 274:19338–19346
Fischer-Parton S, Parton RM, Hickey PC, Dijksterhuis J, Atkinson HA, Read ND (2000) Confocal microscopy of FM4-64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J Microsc 198:246–259
Foissner I, Klima A (2008) Constitutive endocytosis in characean internodal cells is independent of an intact actin cytoskeleton. Cell Biol Int 32:579–580
Foissner I, Wasteneys GO (2007) Wide-ranging effects of eight cytochalasins and latrunculin A and B on intracellular motility and actin filament reorganization in characean internodal cells. Plant Cell Physiol 48:585–597
Foissner I, Menzel D, Wasteneys GO (2009) Microtubule-dependent motility and orientation of the cortical endoplasmic reticulum in elongating characean internodal cells. Cell Motil Cytoskeleton 66:142–155
Galili G, Sengupta-Gopalan C, Ceriotti A (1998) The endoplasmic reticulum of plant cells and its role in protein maturation and biogenesis of oil bodies. Plant Mol Biol 38:1–29
Gocze PM, Freeman DA (1994) Factors underlying the variability of lipid droplet fluorescence in MA-10 leydig tumor cells. Cytometry 17:151–158
Grabski S, de Feijter AW, Schindler M (1993) Endoplasmic reticulum forms a dynamic continuum for lipid diffusion between contiguous soybean root cells. Plant Cell 5:25–38
Greenspan P, Fowler SD (1985) Spectrofluorometric studies of the lipid probe, Nile red. J Lipid Res 26:781–789
Grolig F, Pierson ES (2000) Cytoplasmic streaming: from flow to track. In: Staiger C, Baluska F, Volkmann D, Barlow P (eds) Actin: a dynamic framework for multiple plant cell functions. Kluwer, Dordrecht, pp 165–181
Hsieh K, Huang AHC (2005) Lipid-rich tapetosomes in Brassica tapetum are composed of oleosin-coated oil droplets and vesicles, both assembled in and then detached from the endoplasmic reticulum. Plant J 43:889–899
Huang C-Y, Chung C-I, Lin Y-C, Hsing Y-IC, Huang AHC (2009) Oil bodies and oleosins in Physcomitrella possess characteristics representative of early trends in evolution. Plant Physiol 150:1192–1203
Jarosch R (1961) Das Characeen-Protoplasma und seine Inhaltskörper. Protoplasma 53:34–56
Kamisaka Y, Noda N (2001) Intracellular transport of phosphatidic acid and phosphatidylcholine into lipid bodies in an oleaginous fungus. Mortierella ramanniana var. angulispora. J Biochem 129:19–26
Kamiya N, Kuroda K (1957) Cell operation in Nitella. II. behaviour of isolated endoplasm. Proc Japan Acad 33:201–205
Kean LS, Fuller RS, Nichols JW (1993) Retrograde lipid traffic in yeast: identification of two distinct pathways for internalization of fluorescent-labeled phosphatidylcholine from the plasma membrane. J Cell Biol 123:1403–1419
Klima A, Foissner I (2008) FM dyes label sterol-rich plasma membrane domains and are internalized independently of the cytoskeleton in characean internodal cells. Plant Cell Physiol 49:1508–1521
Knebel W, Quader H, Schnepf E (1990) Mobile and immobile endoplasmic reticulum in onion bulb epidermis cells: short- and long-term observations with a confocal laser scanning microscope. Eur J Cell Biol 52:328–340
Kuerschner L, Moessinger C, Thiele C (2008) Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic 9:338–352
Langhans M, Niemes S, Pimpl P, Robinson DG (2009) Oryzalin bodies: in addition to its anti-microtubule properties, the dinitroaniline herbicide oryzalin causes nodulation of the endoplasmic reticulum. Protoplasma 236:73–84
Lang-Pauluzzi I (2000) The behaviour of the plasma membrane during plasmolysis: a study by UV microscopy. J Microsc 198:188–198
Levine T (2004) Short-range intracellular trafficking of small molecules across endoplasmic reticulum junctions. Trends Cell Biol 14:483–490
Lichtscheidl I, Baluska F (2000) Motility of endoplasmic reticulum in plant cells. In: Staiger C, Baluska F, Volkmann D, Barlow P (eds) Actin: a dynamic framework for multiple plant cell functions. Kluwer, Dordrecht, pp 191–201
Lisboa S, Scherer GEF, Quader H (2008) Localized endocytosis in tobacco pollen tubes: visualisation and dynamics of membrane retrieval by a fluorescent phospholipid. Plant Cell Rep 27:21–28
Martin S, Parton RG (2006a) Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol 7:373–378
Martin S, Parton RG (2006b) Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol 7:373–378
Meshulam T, Herscovitz H, Casavant D, Bernardo J, Roman R, Haugland RP, Strohmeier GS, Diamond RD, Simons ER (1992) Flow cytometric kinetic measurements of neutrophil phospholipase A activation. J Biol Chem 267:21465–21470
Morrot G, Zachowski A, Devaux PF (1990) Partial purification and characterization of the human Mg2+-ATPase: a candidate aminophospholipid translocase. FEBS Lett 266:19–38
Murphy DJ (2001) The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Progr Lipid Res 40:325–438
Murphy DJ, Vance J (1999) Mechanisms of lipid-body formation. Trends Biochem Sci 24:109–115
Murphy DJ, Patel K, Hernandez-Pinzon I (2001) Role of lipid bodies and lipid-body proteins in seeds and other tissues. J Plant Physiol 158:471–478
Oparka KJ (1994) Plasmolysis: new insights into an old process. New Phytol 26:571–591
Quader H, Hofmann A, Schnepf E (1989) Reorganization of the endoplasmic reticulum in epidermal cells of onion bulb scales after cold stress: involvement of cytoskeletal elements. Planta 177:273–280
Saito K, Kuga-Uetake Y, Saito M (2004) Acidic vesicles in living hyphae of an arbuscular mycorrhizal fungus, Gigaspora margarita. Plant Soil 261:231–237
Sakano K, Tazawa M (1986) Tonoplast origin of the envelope membrane of cytoplasmic droplets prepared from Chara internodal cells. Protoplasma 131:247–249
Scherer GFE, Paul RU, Holk A, Martinec J (2002) Down-regulation by elicitors of phosphatidylcholine-hydrolyzing phospholipase C and up-regulation of phospholipase A in plant cells. Biochem Biophys Res Commun 293:766–770
Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, Bland JM, Rothstein SJ, Mullen RT, Dyer JM (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18:2294–2313
Staehelin LA (1997) The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J 11:1151–1165
Terasaki M (1994) Labeling of the endoplasmic reticulum with DiOC6(3). In: Celis JE (ed) Cell biology: a laboratory handbook. Academic, New York, pp 381–386
Thiele C, Spandl J (2008) Cell biology of lipid droplets. Current Opin Cell Biol 20:378–385
Zweytick D, Athenstaedt K, Daum G (2000) Intracellular lipid particles of eukaryotic cells. Biochim Biophys Acta Rev Biomembranes 1469:101–120
Acknowledgements
I am grateful to Patric Schmölzer and Christian Pritz for valuable discussion.
Conflict of interest
The author declares that she has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1
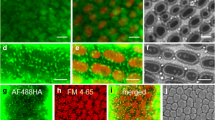
Bodipy-PC-stained cortical ER in an internodal cell of C. corallina before (a) and after 1 h treatment with 50 µM IPC (b). Bar is 8 µm (JPEG 618 kb)
Suppl. Movie 1
Mature C. corallina internodal cell stained with Bodipy PC (green) for 2 h. During this time series, the focus was manually changed from the cortical to the inner cytoplasm which are separated from each other by the stationary layer of autofluorescent chloroplasts (red). Bright green spots visible at the upper and lower side of images 1–10 are Bodipy-PC-stained epiphytes on the outer surface of the cell wall. The horizontal axis of the images corresponds to the long axis of the internode. Note striking contrast in the dynamics of the cortical and subcortical ER. Images were collected at 600-ms interval and played back at 7 fps. Total elapsed time is 27 s (AVI 8645 kb)
Suppl. Movie 2
Bodipy-PC-stained cortical ER and lipid droplets from a mature internodal cell. Time-lapse images were collected at 6.5-s intervals and played back at 5 fps. Total elapsed time is 248 s (AVI 30424 kb)
Suppl. Movie 3
Bodipy-PC-stained cortical ER and lipid droplets from a rapidly elongating internodal cell (compare Fig. 5l–p). Time-lapse images are positioned with horizontal sides parallel to the long axis of the internode. They were collected at 8.8-s intervals and played back at 5 fps. Total elapsed time is 106 s (AVI 3617 kb)
Bodipy-PC-stained cortical ER and lipid droplets from an internodal cell that had recently stopped elongation (compare Fig. 5e–k). Time-lapse images were positioned with horizontal sides slightly oblique to the long axis of the internode, were collected at 7.7-s intervals and played back at 5 fps. Total elapsed time is 453 s (AVI 34722 kb)
Suppl. Movie 5
Bodipy-PC-stained cortical ER and lipid droplets from a mature internodal cell treated with 5 µM CD for 30 min. Time-lapse images were collected at 4.6-s intervals and played back at 5 fps. Total elapsed time is 109 s (AVI 19504 kb)
Suppl. Movie 6
Z-series through cytoplasmic droplet of unstained cell squeezed into Bodipy-PC-containing perfusion solution. Note reticulate meshwork and intense labelling of the nuclear (N) surface. Bodipy fluorescence is absent from chloroplasts (not seen but their positions are indicated by C). Optical sections were taken at 1.03-µm intervals, total stack size is 8.2 µm (AVI 6916 kb)
Rights and permissions
About this article
Cite this article
Foissner, I. Fluorescent phosphocholine—a specific marker for the endoplasmic reticulum and for lipid droplets in Chara internodal cells. Protoplasma 238, 47–58 (2009). https://doi.org/10.1007/s00709-009-0072-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-009-0072-5