Abstract
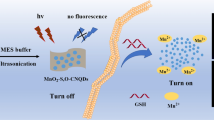
A novel stimulus-responsive surface-enhanced Raman scattering (SERS) nanoprobe has been developed for sensitive glutathione (GSH) detection based on manganese dioxide (MnO2) core and silver/gold nanoparticles (Ag/Au NPs). The MnO2 core is not only capable to act as a scaffold to amplify the SERS signal via producing “hot spots”, but also can be degraded in the presence of the target and thus greatly enhance the nanoprobe sensitivity for sensing of GSH. This approach enables a wide linear range from 1 to 100 µM with a 2.95 µM (3σ/m) detection limit. Moreover, the developed SERS nanoprobe represents great possibility in both sensitive detection of intracellular GSH and even can monitor the change of intracellular GSH level when the stimulant occurs. This sensing system not merely offers a novel strategy for sensitive sensing of GSH, but also provides a new avenue for other biomolecules detection.
Graphical Abstract







Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Wang D, Meng Y, Zhang Y, Wang Q, Lu W, Shuang S, Dong C (2022) A specific discriminating GSH from Cys/Hcy fluorescence nanosensor: the carbon dots-MnO2 nanocomposites. Sens Actuators B: Chem 367:132135
Wang X, Li H, Liu C, Hu Y, Li M, Wu Y (2021) Simple turn-on fluorescent sensor for discriminating Cys/Hcy and GSH from different fluorescent signals. Anal Chem 93(4):2244–2253
Shen Y, Yue J, Shi W, Xu W, Xu S (2020) Target-triggered hot spot dispersion for cellular biothiol detection via background-free surface-enhanced Raman scattering tags. Biosens Bioelectron 151:111957
Zhou Z, Li P, Liu Z, Wu C, Zhang Y, Li H (2022) Construction of a unique fluorescent probe for rapid and highly sensitive detection of glutathione in living cells and zebrafish. Talanta 243:123364
Chen H, Tang Y, Gui J, Li P, Zhu X, Wu C, Yin P, Liu M, Zhang Y, Yao S (2023) Detection and bioimaging of glutathione in cells via a turn-on near-infrared fluorescent nanoprobe composed of Ag2S quantum dots and CoOOH nanosheets. ACS Appl Nano Mater 6(3):1870–1879
Song Z, Dai X, Li M, Teng H, Song Z, Xie D, Luo X (2018) Biodegradable nanoprobe based on MnO2 nanoflowers and graphene quantum dots for near infrared fluorescence imaging of glutathione in living cells. Microchim Acta 185:482
Yuan D, Ding L, Sun Z, Li X (2018) MRI/Fluorescence bimodal amplification system for cellular GSH detection and tumor cell imaging based on manganese dioxide nanosheet. Sci REP-UK 8:1747
Wang Y, Jiang L, Leng Q, Wu Y, He X, Wang K (2016) Electrochemical sensor for glutathione detection based on mercury ion triggered hybridization chain reaction signal amplification. Biosens Bioelectron 77:914–920
Zhong Q, Chen Y, Su A, Wang Y (2018) Synthesis of catalytically active carbon quantum dots and its application for colorimetric detection of glutathione. Sens Actuators B: Chem 273:1098–1102
Yao C, Wang J, Zheng A, Wu L, Zhang X, Liu X (2017) A fluorescence sensing platform with the MnO2 nanosheets as an effective oxidant for glutathione detection. Sens Actuators B: Chem 252:30–36
Li Y, Jiang L, Zou Y, Song Z, Jin S (2021) Highly reproducible SERS sensor based on self-assembled Au nanocubic monolayer film for sensitive and quantitative detection of glutathione. Appl Surf Sci 540(2):148381
Shu Y, Gao J, Chen J, Yan J, Sun J, Jin D, Xu Q, Hu X (2021) A near-infrared fluorescent sensor based on the architecture of low-toxic Ag2S quantum dot and MnO2 nanosheet for sensing glutathione in human serum sample. Talanta 221:121475
He X, Ge C, Zheng X, Tang B, Chen L, Li S, Wang L, Zhang L, Xu Y (2020) Rapid identification of alpha-fetoprotein in serum by a microfluidic SERS chip integrated with Ag/Au Nanocomposites. Sens Actuators B: Chem 317:128196
Kneipp J, Kneipp H, Kneipp K (2008) SERS-a single-molecule and nanoscale tool for bioanalytics. Chem Soc Rev 37(5):1052–1060
Tang J, Chen W, Ju H (2019) Rapid detection of pesticide residues using a silver nanoparticles coated glass bead as nonplanar substrate for SERS sensing. Sens Actuators B: Chem 287:576–583
Wang T, Dong P, Zhu C, Gao W, Sha P, Wu Y, Wu X (2021) Fabrication of 2D titanium carbide MXene/Au nanorods as a nanosensor platform for sensitive SERS detection. Ceram Int 47(21):30082–30090
Zhu Y, Wu J, Wang K, Xu H, Qu M, Gao Z, Guo L, Xie J (2021) Facile and sensitive measurement of GSH/GSSG in cells by surface-enhanced Raman spectroscopy. Talanta 224:121852
Dai X, Song Z, Song W, Zhang J, Fan G, Wang W, Luo X (2020) Shell-switchable SERS blocking strategy for reliable signal-on SERS sensing in living cells: detecting an external target without affecting the internal Raman molecule. Anal Chem 92(16):11469–11475
Dai X, Lu L, Zhang X, Song Z, Song W, Chao Q, Li Q, Wang W, Chen J, Fan G (2021) MnO2 shell-isolated SERS nanoprobe for the quantitative detection of ALP activity in trace serum: relying on the enzyme-triggered etching of MnO2 shell to regulate the signal. Sens Actuators B: Chem 334:129605
Li P, Zhou B, Ge M, Jing X, Yang L (2022) Metal coordination induced SERS nanoprobe for sensitive and selective detection of histamine in serum. Talanta 237:122913
Su S, Zhang C, Yuwen L, Chao J, Zuo X, Liu X, Song C, Fan C, Wang L (2014) Creating SERS hot spots on MoS2 nanosheets with in situ grown gold nanoparticles. ACS Appl Mater Interfaces 6(21):18735–18741
Yun BJ, Koh W (2020) Highly-sensitive SERS-based immunoassay platform prepared on silver nanoparticle-decorated electrospun polymeric fibers. J Ind Eng Chem 82:341–348
Liao W, Liu K, Chen Y, Hu J, Gan Y (2021) Au-Ag bimetallic nanoparticles decorated silicon nanowires with fixed and dynamic hot spots for ultrasensitive 3D SERS sensing. J Alloys Compd 868:159136
Wang K, Sun D, Pu H, Wei Q (2019) Shell thickness-dependent Au@Ag nanoparticles aggregates for high-performance SERS applications. Talanta 195:506–515
Peña-Rodríguez O, Pal U (2011) Enhanced plasmonic behavior of bimetallic (Ag-Au) multilayered spheres. Nanoscale Res Lett 6:279
Yu J, Ma Y, Yang C, Zhang H, Liu L, Su J, Gao Y (2018) SERS-active composite based on rGO and Au/Ag core-shell nanorods for analytical applications. Sens Actuators B: Chem 254:182–188
Xue H, Yu M, He K, Liu Y, Cao Y, Shui Y, Li J, Farooq M, Wang L (2020) A novel colorimetric and fluorometric probe for biothiols based on MnO2 NFs-rhodamine B system. Anal Chim Acta 1127:39–48
Liu W, Zhang K, Zhuang L, Liu J, Zeng W, Shi J, Zhang Z (2019) Aptamer/photosensitizer hybridized mesoporous MnO2 based tumor cell activated ROS regulator for precise photodynamic therapy of breast cancer. Colloids Surf B 184:110536
Nawaz F, Xie Y, Cao H, Xiao J, Zhang X, Li M, Duan F (2015) Catalytic ozonation of 4-nitrophenol over an mesoporous α-MnO2 with resistance to leaching. Catal Today 258:595–601
Poudel BK, Gupta B, Ramasamy T, Thapa RK, Pathak S, Oh KT, Jeong J, Choi H, Yong CS, Kim JO (2017) PEGylated thermosensitive lipid-coated hollow gold nanoshells for effective combinational chemo-photothermal therapy of pancreatic cancer. Colloids Surf B 160:73–83
Zhang H, Lin X, Wang A, Zhao Y, Chu H (2015) Fluorescence enhancement of europium complexes by core-shell Ag@SiO2 nanoparticles. Spectrochim Acta, Part A 151:716–722
Pham TTH, Vu XH, Dien ND, Trang TT, Van Truong N, Thanh TD, Tan PM, Ca NX (2020) The structural transition of bimetallic Ag-Au from core/shell to alloy and SERS application. RSC Adv 10:24577–24594
Han L, Liu SG, Liang JY, Li NB, Luo HQ (2019) Free-label dual-signal responsive optical sensor by combining resonance Rayleigh scattering and colorimetry for sensitive detection of glutathione based on ultrathin MnO2 nanoflakes. Sens Actuators B: Chem 288:195–201
Li Y, Zhang L, Zhang Z, Liu Y, Chen J, Liu J, Du P, Guo H, Lu X (2021) MnO2 nanospheres assisted by cysteine combined with MnO2 nanosheets as a fluorescence resonance energy transfer system for “switch-on” detection of glutathione. Anal Chem 93(27):9621–9627
Ścibior D, Skrzycki M, Podsiad M, Czeczot H (2008) Glutathione level and glutathione-dependent enzyme activities in blood serum of patients with gastrointestinal tract tumors. Clin biochem 41:852–858
Lu Z, Lu Y, Fan C, Sun X, Zhang M, Lu Y (2018) A two-separated-emission fluorescent probe for simultaneous discrimination of Cys/Hcy and GSH upon excitation of two different wavelengths. J Mater Chem B 6(48):8221–8227
Zhang Y, Shao X, Wang Y, Pan F, Kang R, Peng F, Huang Z, Zhang W, Zhao W (2015) Dual emission channels for sensitive discrimination of Cys/Hcy and GSH in plasma and cells. Chem Commun 51(20):4245–4248
Hu Q, Sun H, Zhou X, Gong X, Xiao L, Liu L, Yang Z (2020) Bright-yellow-emissive nitrogen-doped carbon nanodots as a fluorescent nanoprobe for the straightforward detection of glutathione in food samples. Food Chem 325:126946
Zhu J, Xia T, Cui Y, Yang Y, Qian G (2019) A turn-on MOF-based luminescent sensor for highly selective detection of glutathione. J Solid State Chem 270:317–323
Su P, Zhu Z, Tian Y, Liang L, Wu W, Cao J, Cheng B, Liu W, Tang Y (2020) A TAT peptide-based ratiometric two-photon fluorescent probe for detecting biothiols and sequentially distinguishing GSH in mitochondria. Talanta 218:121127
Zhang B, Zou H, Qi Y, Zhang X, Sheng R, Zhang Y, Sun R, Chen L, Lv R (2021) Assembly of polyoxometalates/polydopamine nanozymes as a multifunctional platform for glutathione and Escherichia coli O157: H7 detection. Microchem J 164:106013
Sun R, Lv R, Zhang Y, Du T, Li Y, Chen L, Qi Y (2022) Colorimetric sensing of glucose and GSH using core-shell Cu/Au nanoparticles with peroxidase mimicking activity. RSC Adv 12:21875–21884
Zhao X, Wu K, Lyu H, Zhang X, Liu Z, Fan G, Zhang X, Zhu X, Liu Q (2019) Porphyrin functionalized Co(OH)2/GO nanocomposites as an excellent peroxidase mimic for colorimetric biosensing. Analyst 144(7):5284–5291
Jiang C, Zhang C, Song J, Ji X, Wang W (2021) Cytidine-gold nanoclusters as peroxidase mimetic for colorimetric detection of glutathione (GSH), glutathione disulfide (GSSG) and glutathione reductase (GR). Spectrochim Acta, Part A 250:119316
Chen X, Batist G (1998) Sensitization effect of L-2-oxothiazolidine-4-carboxylate on tumor cells to melphalan and the role of 5-oxo-L-prolinase in glutathione modulation in tumor cells. Biochem Pharmacol 56(6):743–749
Russo A, DeGraff W, Friedman N, Mitchell JB (1986) Selective modulation of glutathione levels in human normal versus tumor cells and subsequent differential response to chemotherapy drugs. Cancer Res 46(6):2845–2848
Gao G, Jiang Y, Jia H, Yang J, Wu F (2018) On-off-on fluorescent nanosensor for Fe3+ detection and cancer/normal cell differentiation via silicon-doped carbon quantum dots. Carbon 134:232–243
Nur G, Nazıroğlu M, Deveci HA (2017) Synergic prooxidant, apoptotic and TRPV1 channel activator effects of alpha-lipoic acid and cisplatin in MCF-7 breast cancer cells. J Recept Sig Transd 37(6):569–577
Wang C, Yun G, Sen HAZY, Ying W, Yang H (2022) MnO2 coated Au nanoparticles advance SERS detection of cellular glutathione. Biosens Bioelectron 215:114388
Acknowledgements
This work supported by the Natural Science Foundation of Hebei Province (Nos. H2020206416, H2022206320, B2021206005), the Youth Top Talent Project of Hebei Province Higher Education (No. BJ2021050), Chunyu Project Outstanding Youth Fund of Hebei Medical University (No. CYYQ2021003), and the National Natural Science Foundation of China (No. 82204097).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, X., Quan, C., Ren, T. et al. MnO2 nanoparticles decorated with Ag/Au nanotags for label-based SERS determination of cellular glutathione. Microchim Acta 190, 341 (2023). https://doi.org/10.1007/s00604-023-05870-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05870-4