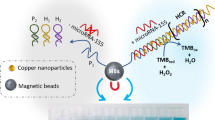
Abstract
An electrochemical strategy based on bimetallic nanozyme in collaboration with toehold-mediated DNA replacement effect is proposed for the sensitive determination of miRNA-21. The AuPt nanoparticles (AuPt NPs) are prepared as a catalytic beacon; it shows favorable peroxidase properties with a Michaelis contant (Km) of 0.072 mM for H2O2, which is capable of catalyzing H2O2 to induce an intense redox reaction, and causing a measurable electrochemical signal. To further enhance the strength of the signal response, a novel toehold-mediated DNA replacement strategy is employed. DNA strands with specific sequences are modified on electrodes and AuPt NPs, respectively. In the presence of miRNA-21, a cyclic substitution effect is subsequently activated via a specific toehold sequence and leads to a large accumulation of AuPt NPs on the electrodes. Subsequently, a strong signal depending on the amount of miRNA-21 is obtained after adding a small amount of H2O2. The analytical range of this determination method is from 0.1 pM to 1.0 nM, and the LOD is 84.1 fM. The spike recoveries for serum samples are 95.0 to 102.4% and the RSD values are 3.7 to 5.8%. The results suggests a promising application of the established method in clinical testing and disease diagnosis.
Graphical Abstract






Similar content being viewed by others

References
Peng XX, Guo T, Lu H et al (2020) Nanostructuring synergetic base-stacking effect: an enhanced versatile sandwich sensor enables ultrasensitive detection of microRNAs in blood. ACS Sens 5:2514–2522. https://doi.org/10.1021/acssensors.0c00772
Huang X, Zhu X, Yu Y et al (2021) Dissecting miRNA signature in colorectal cancer progression and metastasis. Cancer Lett 501:66–82. https://doi.org/10.1016/j.canlet.2020.12.025
Asangani IA, Rasheed SAK, Nikolova DA et al (2008) MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27:2128–2136. https://doi.org/10.1038/sj.onc.1210856
Frankel LB, Christoffersen NR, Jacobsen A et al (2008) Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem 283:1026–1033. https://doi.org/10.1074/jbc.m707224200
Wang ZX, Bian HB, Wang JR et al (2011) Prognostic significance of serum miRNA-21 expression in human non-small cell lung cancer. J Surg Oncol 104:847–851. https://doi.org/10.1002/jso.22008
Qu K, Zhang X, Lin T et al (2017) Circulating miRNA-21-5p as a diagnostic biomarker for pancreatic cancer: evidence from comprehensive miRNA expression profiling analysis and clinical validation. Sci Rep 7:1692. https://doi.org/10.1038/s41598-017-01904-z
Hasanzadeh M, Movahedi M, Rejali M et al (2019) The potential prognostic and therapeutic application of tissue and circulating microRNAs in cervical cancer. J Cell Physiol 234:1289–1294. https://doi.org/10.1002/jcp.27160
Huang Q, Mao Z, Li S et al (2014) A non-radioactive method for small RNA detection by northern blotting. Rice 7:26. https://doi.org/10.1186/s12284-014-0026-1
Tian H, Sun Y, Liu C et al (2016) Precise quantitation of microRNA in a single cell with droplet digital PCR based on ligation reaction. Anal Chem 88:11384–11389. https://doi.org/10.1021/acs.analchem.6b01225
Zhang JL, Yang HL, Liu WJ et al (2022) Rapid 16S rDNA electrochemical sensor for detection of bacteria based on the integration of target-triggered hairpin self-assembly and tripedal DNA walker amplification. Anal. Chim. Acta 1190:339266. https://doi.org/10.1016/j.aca.2021.339266
Hira SA, Nallal M, Rajendran K et al (2020) Ultrasensitive detection of hydrogen peroxide and dopamine using copolymer-grafted metal-organic framework based electrochemical sensor. Anal Chim Acta 1118:26–35. https://doi.org/10.1016/j.aca.2020.04.043
Robert A, Meunier B (2022) How to define a nanozyme. ACS Nano 16:6956–6959. https://doi.org/10.1021/acsnano.2c02966
Xia N, Wang X, Zhou BB et al (2016) Electrochemical detection of amyloid-β oligomers based on the signal amplification of a network of silver nanoparticles. ACS Appl Mater Interfaces 8:19303–19311. https://doi.org/10.1021/acsami.6b05423
Liu LL, Chang Y, Xia N et al (2017) Simple, sensitive and label-free electrochemical detection of microRNAs based on the in situ formation of silver nanoparticles aggregates for signal amplification. Biosens Bioelectron 94:235–242. https://doi.org/10.1016/j.bios.2017.02.041
Shi K, Dou B, Yang C et al (2015) DNA-fueled molecular machine enables enzyme-free target recycling amplification for electronic detection of microRNA from cancer cells with highly minimized background noise. Anal Chem 87:8578–8583. https://doi.org/10.1021/acs.analchem.5b02418
Wei H, Wang E (2013) Nanomaterials with enzyme-like characteristics (nanozymes): next- generation artificial enzymes. Chem Soc Rev 42:6060–6093. https://doi.org/10.1039/C3CS35486E
Wang HY, Xie AJ, Li SJ et al (2022) Three-dimensional g-C3N4/MWNTs/GO hybrid electrode as electro-chemical sensor for simultaneous determination of ascorbic acid, dopamine and uric acid. Anal Chim Acta 1211:339907. https://doi.org/10.1016/j.aca.2022.339907
Zhao RN, Feng Z, Zhao YN et al (2019) A sensitive electrochemical aptasensor for Mucin 1 detection based on catalytic hairpin assembly coupled with PtPd NPs peroxidase-like activity. Talanta 200:503–510. https://doi.org/10.1016/j.talanta.2019.03.012
Chen T, Xu J, Yang P et al (2019) Facile controlled synthesis of AuPd and AuPt bimetallic nanocrystals for enhanced electrocatalytic sensing. Sens Actuators B Chem 298:126724. https://doi.org/10.1016/j.snb.2019.126724
Liu J, Cao L, Huang W et al (2011) Preparation of AuPt alloy foam films and their superior electrocatalytic activity for the oxidation of formic acid. ACS Appl Mater Interfaces 3:3552–3558. https://doi.org/10.1021/am200782x
Liu Y, Xu Q, Zhang Y et al (2021) An electrochemical aptasensor based on AuPt alloy nanoparticles for ultrasensitive detection of amyloid-β oligomers. Talanta 231:122360. https://doi.org/10.1016/j.talanta.2021.122360
Özay B, McCalla ES (2021) A review of reaction enhancement strategies for isothermal nucleic acid amplification reactions 3:100033. https://doi.org/10.1016/j.snr.2021
Guo Q, Yu Y, Zhang H et al (2020) Electrochemical sensing of exosomal microRNA based on hybridization chain reaction signal amplification with reduced false-positive signals. Anal Chem 92:5302–5310. https://doi.org/10.1021/acs.analchem.9b05849
Wang F, Lu CH, Willner I (2014) From cascaded catalytic nucleic acids to enzyme-DNA nanostructures: controlling reactivity, sensing, logic operations, and assembly of complex structures. Chem Rev 114:2881–2941. https://doi.org/10.1021/cr400354z
Wang L, Zhang L, Yu Y et al (2021) DNA cyclic assembling control in an electrochemical strategy with MoS2@AuNPs for determination of kanamycin. Microchim Acta 188:264. https://doi.org/10.1007/s00604-021-04916-9
Wang S, Emery JN, Liu PA (2019) A novel synthetic toehold switch for microRNA detection in mammalian cells. ACS Synth Biol 8:1079–1088. https://doi.org/10.1021/acssynbio.8b00530
Miao P, Tang YG (2021) Cascade toehold-mediated strand displacement reaction for ultrasensitive detection of exosomal microRNA. CCS Chem 3:2331–2339. https://doi.org/10.31635/ccschem.020.202000458
Xu SH, Wang YL, Yao YY et al (2022) Toehold-mediated strand displacement coupled with single nanoparticle dark-field microscopy imaging for ultrasensitive biosensing. Nanoscale 14:3496–3503. https://doi.org/10.1039/d1nr08030j
Liang KL, Wang H, Li P et al (2020) Detection of microRNAs using toehold-initiated rolling circle amplification and fluorescence resonance energy transfer. Talanta 207:120285. https://doi.org/10.1016/j.talanta.2019.120285
Weng X, Liu Y, Xue Y et al (2017) L-Proline bio-inspired synthesis of AuPt nanocalliandras as sensing platform for label-free electrochemical immunoassay of carbohydrate antigen. Sens Actuators B Chem 250:61–68. https://doi.org/10.1016/j.snb.2017.04.156
Feng J, He LL, Fang R et al (2016) Bimetallic PtAu superlattice arrays: highly electroactive and durable catalyst for oxygen reduction and methanol oxidation reactions. J Power Sources 330:140–148. https://doi.org/10.1016/j.jpowsour.2016.08.094
Ren Y, Li C, Li B et al (2021) PtPd nanoframes derived from Pd@ PdPt core-shell rhombic dodecahedrals with excellent catalytic performance toward methanol oxidation. Inorg Chem Front 8:2280–2287. https://doi.org/10.1039/D1QI00081K
Khan IA, Sofian M, Badshah A et al (2020) Stable and efficient PtRu electrocatalysts supported on Zn-BTC MOF derived microporous carbon for formic acid fuel cells application. Front Chem 8:367. https://doi.org/10.3389/fchem.2020.00367
Fu X, Wan C, Zhang A et al (2020) Pt3Ag alloy wavy nanowires as highly effective electrocatalysts for ethanol oxidation reaction. Nano Res 13:1472–1478. https://doi.org/10.1007/s12274-020-2754-4
Nivedita B, Thu HH, Guillon FX et al (2019) Coupling electrochemical adsorption and long-range electron transfer: label-free DNA mismatch detection with ultramicroelectrode (UME). Electroanalysis 31:2232–2237. https://doi.org/10.1002/elan.201900357
Ferapontova EE (2019) Electron transfer in DNA at electrified interfaces. Chem Asian J 14:3773–3781. https://doi.org/10.1002/asia.201901024
Tan Z, Cao L, He X et al (2020) A label-free immunosensor for the sensitive detection of hepatitis B e antigen based on PdCu tripod functionalized porous graphene nanoenzymes. Bioelectrochem 133:107461. https://doi.org/10.1016/j.bioelechem.2020.107461
Xian Z, Zhang L, Yu Y et al (2021) Nanozyme based on CoFe2O4 modified with MoS2 for colorimetric determination of cysteine and glutathione. Microchim Acta 188:65. https://doi.org/10.1007/s00604-021-04702-7
Funding
This work is supported by the National Natural Science Foundation of China (Nos. 21575043, 52070080, 22004039, and 22206069), the Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515110256), Science and Technology Projects Foundation (Basic and Applied Basic Research) in Guangzhou (Nos. 202102020043 and 202102080612), and Foundation of Department of Education of Guangdong Province (No. 2020KTSCX033).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, Z., Zhang, L., Yu, Y. et al. An electrochemical determination strategy for miRNA based on bimetallic nanozyme and toehold-mediated DNA replacement procedure. Microchim Acta 190, 149 (2023). https://doi.org/10.1007/s00604-023-05720-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05720-3