Abstract
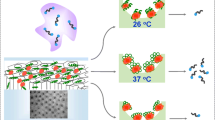
A strategy for preparing a dual-stimuli-responsive porous polymer membrane enzyme reactor (D-PPMER) is described, consisting of poly (styrene-maleic anhydride-N-isopropylacrylamide-acrylate-3′,3′-dimethyl-6-nitro-spiro[2H-1-benzopyran-2,2′-indoline]-1′-esterspiropyran ester) [P(S-M–N-SP)] and D-amino acid oxidase. Tunable control via “on/off” 365 nm UV light irradiation and temperature variation was used to change the membrane surface configuration and adjust the enzymolysis efficiency of the D-PPMER. A chiral capillary electrophoresis technique was developed for evaluation of the enzymatic efficiency of D-PPMER with a Zn(II)-dipeptide complex as the chiral selector and D,L-serine as the substrate. Interestingly, the enzymatic kinetic reaction rate of D-PPMER under UV irradiation at 36 °C (9.2 × 10−2 mM·min−1) was 3.2-fold greater than that of the free enzyme (2.9 × 10−2 mM·min−1). This was because upon UV irradiation at high temperature, the P(SP) and P(N) moieties altered from a “stretched” to a “curled” state to encapsulate the enzyme in smaller cavities. The confinement effect of the cavities further improved the enzymatic efficiency of the D-PPMER. This protocol highlights the outstanding potential of smart polymers, enables tunable control over the kinetic rates of stimuli-responsive enzyme reactors, and establishes a platform for adjusting enzymolysis efficiency using two different stimuli.
Graphical abstract





Similar content being viewed by others
References
Tang RH, Liu LN, Zhang SF, Li A, Li ZD (2015) Modification of a nitrocellulose membrane with cellulose nanofibers for enhanced sensitivity of lateral flow assays: application to the determination of Staphylococcus aureus. Microchim Acta 186:831
Cox JA, Hensley PM, Loch CL (2003) Evaluation of polycation-stabilized lactate oxidase in a silica sol-gel as a biosensor platform. Microchim Acta 142:1–5
Zheng J, Strutzenberg TS, Reich A, Dharmarajan V, Pascal BG, Crynen GC, Novick SJ, Garcia-Ordonez RD, Griffin PR (2020) Comparative analysis of cleavage specificities of immobilized porcine pepsin and nepenthesin II under hydrogen/deuterium exchange conditions. Anal Chem 92:11018–11028
Califano V, Costantini A (2018) Immobilization of cellulolytic enzymes in mesostructured silica materials. Catalysts 6:3200–3218
Qiao J, Kim J, Wang Y, Qi L, Wang F, Moon M (2016) Trypsin immobilization in ordered porous polymer membranes for effective protein digestion. Anal Chim Acta 906:156–164
Matsuura SI, Ikeda T, Chiba M, Yamamoto K (2021) Efficient production of gamma-aminobutyric acid by glutamate decarboxylase immobilized on an amphiphilic organic-inorganic hybrid porous material. J Biosci Bioeng 131:250–255
Spuhler PS, Sola L, Zhang X, Monroe MR, Greenspun JT, Chiari M, Uenlue MS (2012) Precisely controlled smart polymer scaffold for nanoscale manipulation of biomolecules. Anal Chem 84:10593–10599
Lei L, Liu J, Ma X, Yang H, Lei Z (2019) A novel strategy to synthesize dual-responsive polymeric nanocarriers for investigating the activity and stability of immobilized pectinase. Biotechno Appl Biochem 66:376–388
Qiao J, Jiang J, Liu L, Shen J, Qi L (2019) Enzyme reactor based on reversible pH-controlled catalytic polymer porous membrane. ACS Appl Mater Interfaces 11:15133–15140
Grimm O, Massmann SC, Schacher FH (2019) Synthesis and solution behaviour of dual light- and temperature-responsive poly(triethylene glycolco-spiropyran) copolymers and block copolymers. Polym Chem 10:2674–2685
Schoffelen S, van Hest JCM (2012) Multi-enzyme systems: bringing enzymes together in vitro. Soft Matter 8:1736–1746
Koeller KM, Wong CH (2000) Synthesis of complex carbohydrates and glycoconjugates: enzyme-based and programmable one-pot strategies. Chem Rev 100:4465–4493
Thibodeaux CJ, Melancon CE, Liu HW (2007) Unusual sugar biosynthesis and natural product glycodiversification. Nature 446:1008–1016
Ji Q, Wang B, Tan J, Zhu L, Li L (2016) Immobilized multienzymatic systems for catalysis of cascade reactions. Process Biochem 51:1193–1203
Li X, Zhou L, Wei Y, El-Toni AM, Zhang F, Zhao D (2015) Anisotropic encapsulation-induced synthesis of asymmetric single-hole mesoporous nanocages. J Am Chem Soc 137:5903–5906
Cong Y, Li Q, Chen M, Wu L (2017) Synthesis of dual-stimuli-responsive microcontainers with two payloads in different storage spaces for preprogrammable release. Angew Chem Int Ed 56:3552–3556
Ge Z, Liu SY (2013) Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem Soc Rev 42:7298–7325
Han J, Luo P, Wang L, Wu J, Li C, Wang Y (2020) Construction of a multienzymatic cascade reaction system of co-immobilized hybrid nanoflowers for efficient conversion of starch into gluconic acid. ACS Appl Mater Interfaces 12:15023–15033
Huang J, Qin H, Wang B, Tan Q, Lu J (2019) Design of smart polyacrylates showing thermo-, pH-, and CO2-responsive features. Polym Chem 10:6379–6384
Benoit C, Talitha S, David F, Michel S, Anna SJ, Rachel AV, Patrice W (2017) Dual thermo- and light-responsive coumarin based copolymers with programmable cloud points. Polym Chem 8:4512–4519
Liu X, Gong X, Yuan J, Fan X, Zhang X, Ren T, Yang S, Yang R, Yuan L, Zhang XB (2021) Dual-stimulus responsive near-infrared reversible ratiometric fluorescent and photoacoustic probe for in vivo tumor imaging. Anal Chem 93:5420–5429
He T, He J, Younis MR, Blum NT, Lei S, Zhang Y, Huang P, Lin J (2021) Dual-stimuli-responsive nanotheranostics for dual-targeting photothermal-enhanced chemotherapy of tumor. ACS Appl Mater Interfaces 13:22204–22212
Ding Y, Xu S, Zhang H, Zhang J, Qiu Z, Chen H, Wang J, Zheng J, Wu J (2021) One-step fabrication of a micro/nanosphere-coordinated dual stimulus-responsive nanofibrous membrane for intelligent antifouling and ultrahigh permeability of viscous water-in-oil emulsions. ACS Appl Mater Interfaces 13:27635–27644
Chen SY, Chen MJ, Yang JF, Zeng XQ, Zhou YB, Yang S, Yang RH, Yuan Q, Zheng J (2021) Design and engineering of hypoxia and acidic pH dual-stimuli-responsive intelligent fluorescent nanoprobe for precise tumor imaging. Small 17:2100243
Zhou ZH, Zhang JG, Chen Q, Luo YL, Xu F, Chen YS (2020) Temperature and photo dual-Stimuli responsive block copolymer self-assembly micelles for cellular controlled drug release. Macromol Biosci 21:2000291
Xue XQ, Yang J, Huang WY, Yang HJ, Jiang BB, Li F, Jiang Y (2015) Dual thermo- and light-responsive nanorods from self-assembly of the 4-propoxyazobenzene-terminated poly(N-isopropylacrylamide) in aqueous solution. Polymer 73:195–204
Zhang X, Zhang PP, Lu M, Qi DM, Muller-Buschbaum P, Zhong Q (2021) Synergistic stain removal achieved by controlling the fractions of light and thermo responsive components in the dual-responsive copolymer immobilized on cotton fabrics by cross-linker. ACS Appl Mater Interfaces 13:27372–27381
Liu T, Liu SY (2011) Responsive polymers-based dual fluorescent chemosensors for Zn2+ Ions and temperatures working in purely aqueous media. Anal Chem 84:2775–2785
Schattling P, Jochum FD, Theato P (2014) Multi-stimuli responsive polymers-the all-in-one talents. Polym Chem 5:25–36
Zhang QL, Schattling P, Theato P, Hoogenboom R (2015) UV-tunable upper critical solution temperature behavior of azobenzene containing poly(methyl methacrylate) in aqueous ethanol. Eur Polym J 62:435–441
Narayanan A, Chandel S, Ghosh N, De P (2015) Visualizing phase Ttransition behavior of dilute stimuli responsive polymer solutions via mueller matrix polarimetry. Anal Chem 87:9120–9125
Malmstadt N, Yager P, Hoffman AS, Stayton PS (2003) A smart microfluidic affinity chromatography matrix composed of poly(N-isopropylacrylamide)-coated beads. Anal Chem 75:2943–2949
Cole MA, Jasieniak M, Thissen H, Voelcker NH, Griesser HJ (2009) Time-of-flight-secondary ion mass spectrometry study of the temperature dependence of protein adsorption onto poly(N-isopropylacrylamide) graft coatings. Anal Chem 81:6905–6912
Cheng HB, Zhang SC, Qi J, Liang XJ, Yoon J (2021) Advances in application of azobenzene as a trigger in biomedicine: molecular design and spontaneous assembly. Adv Mater 33:2007290
Yu C, Li L, Hu P, Yang Y, Wei W, Deng X, Wang L, Tay FR, Ma J (2021) Recent advances in stimulus-responsive nanocarriers for gene therapy. Adv Sci 8:2100540
Ghani M, Heiskanen A, Kajtez J, Rezaei B, Larsen NB, Thomsen P, Kristensen A, Zukauskas A, Alm M, Emneus J (2021) On-demand reversible UV-triggered Interpenetrating polymer network-based drug delivery system using the spiropyran-merocyanine hydrophobicity switch. ACS Appl Mater Interfaces 13:3591–3604
Funding
This work is supported by the National Natural Science Foundation of China (Nos. 21874138, 21727809, 21635008, and 22074148).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shen, J., Qiao, J., Zhang, X. et al. Dual-stimuli-responsive porous polymer enzyme reactor for tuning enzymolysis efficiency. Microchim Acta 188, 435 (2021). https://doi.org/10.1007/s00604-021-05095-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05095-3