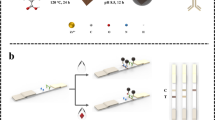
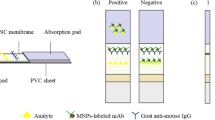
Abstract
A high-affinity monoclonal antibody (mAb) has been prepared and separately a gold nanoparticle (AuNP)-based and a near-infrared (NIR) fluorescence-based lateral flow immunoassay (LFA) developed for determination of 5-hydroxyflunixin residue in raw milk. The AuNP and IRDye® 800CW were used to label anti-5-hydroxyflunixin mAb to form the AuNP-mAb and NIR dye-mAb conjugates, respectively. Quantitative determination of 5-hydroxyflunixin was achieved by imaging the optical or fluorescence intensity of the AuNP-mAb and NIR dye-mAb captured on the test line. As a result, the detection limits of the AuNP-based LFA and NIR dye-based LFA were 0.82 and 0.073 ng/mL in raw milk, respectively. The considerable improvement on assay sensitivity of the NIR-based LFA can be attributed to the lower background and less antibody consumption per test than that of the AuNP-based LFA. The spiking experiment by the NIR-based LFA yielded 85.7–112.6% recovery with a relative standard deviation below 14%, indicating that it has satisfactory assay accuracy and precision. Furthermore, the analytical results of actual samples by the NIR dye-based LFA were consistent with that by instrumental analysis. Therefore, these results demonstrated that the NIR dye is an ideal alternative label to the conventional AuNP for the development of LFA for veterinary drugs in animal-origin food.

Graphical abstract




Similar content being viewed by others
References
Pairis-Garcia MD, Karriker LA, Johnson AK, Kukanich B, Wulf L, Sander S, Millman ST, Stalder KJ, Coetzee JF (2013) Pharmacokinetics of flunixin meglumine in mature swine after intravenous, intramuscular and oral administration. BMC Vet Res 9:1–7
Schering-Plough Animal Health (2005) Supplemental new animal drug application: BANAMINE-S(flunixin meglumine) injectable solution for swine [P]:101–479
Elmas M, Yazar E, Uney K, Er Karabacak A, Traş B (2008) Pharmacokinetics of enrofloxacin and flunixin meglumine and interactions between both drugs after intravenous co-administration in healthy and endotoxaemic rabbits. Vet J 177:418–424
Milovanović M, Vučković S, Prostran M, Trailović S, Jovanović M (2016) L-arginine-no system participates in the analgesic effect of flunixin meglumine in the rat. Acta Vet-Beograd 66:103–114
Zhu A, Peng T, Liu L, Xia X, Hu T, Tao X, Wen K, Cheng L, Li J, Ding S, Cao X, Jiang H (2013) Ultra-performance liquid chromatography-tandem mass spectrometry determination and depletion profile of flunixin residues in tissues after single oral administration in rabbits. J Chromatogr B 934:8–15
EMEA/MRL/661/99-FINAL (1999) Committee for veterinary medicinal products flunixin summary reports (1). https://www.ema.europa.eu/en/documents/mrl-report/flunixin-summary-report-1-committee-veterinary-medicinal-products_en.pdf, Accessed on Dec. 10, 2019
USDA/FSIS, U.S. FSIS (2001) Residue limits for veterinary drugs, food additives and unavoidable contaminants in meat, poultry and egg products. https://www.fsis.usda.gov/wps/wcm/connect/2fe2afb9-b935-4a74-83e0-587c41b2f784/2001_Residue_Limits_Veterinary_Drugs_App4.pdf?MOD=AJPERES, Accessed on Dec. 10, 2019
Ministry of Agriculture of the People’s Republic of China (2017) Announcement No. 2543, Maximum Residue Limit of Flunixin. http://jiuban.moa.gov.cn/sjzz/syj/shenpi/201706/t20170630_5732068.htm, Accessed on Dec. 10, 2019
Belal FF, El-Razeq SAA, Fouad MM, Fouad FA (2015) Micellar high performance liquid chromatographic determination of flunixin meglumine in bulk, pharmaceutical dosage forms, bovine liver and kidney. Anal Chem Res 3:63–69
Liu ZY, Yang K, Chen FH, Long XM, Deng YB, Kuang GW, Sun ZL (2015) Development of a rapid method for the confirmatory analysis of flunixin residue in animal tissues using liquid chromatography–tandem mass spectrometry. Food Anal Methods 8:352–362
Shelver WL, Tell LA, Sarah W, Wetzlich SE, Baynes RE, Riviere JE, Smith DJ (2013) Comparison of ELISA and LC-MS/MS for the measurement of flunixin plasma concentrations in beef cattle after intravenous and subcutaneous administration. J Agric Food Chem 61:2679–2686
Lin L, Jiang W, Xu L, Liu L, Song S, Kuang H (2018) Development of IC-ELISA and immunochromatographic strip assay for the detection of flunixin meglumine in milk. Food Agric Immunol 29:193–203
Chen X, Peng S, Liu C, Zou X, Ke Y, Jiang W (2019) Development of an indirect competitive enzyme-linked immunosorbent assay for detecting flunixin and 5-hydroxyflunixin residues in bovine muscle and milk. Food Agric Immunol 30:320–332
Raeisossadati MJ, Danesh NM, Borna F, Gholamzad M, Ramezani M, Abnous K, Taghdisi SM (2016) Lateral flow based immunobiosensors for detection of food contaminants. Biosens Bioelectron 86:235–246
Gong H, Cradduck M, Cheung L, Michael Olive D (2012) Development of a near-infrared fluorescence ELISA method using tyramide signal amplification. Anal Biochem 426:27–29
Liang G, Liu S, Zou G, Zhang X (2012) Ultrasensitive immunoassay based on anodic near-infrared electrochemiluminescence from dual-stabilizer-capped CdTe nanocrystals. Anal Biochem 84:10645–10649
Swanson C, D’Andrea A (2013) Lateral flow assay with near-infrared dye for multiplex detection. Clin Chem 59:641–648
Zhang F, Zou MQ, Chen Y, Li J, Wang Y, Qi X, Xue Q (2014) Lanthanide-labeled immunochromatographic strips for the rapid detection of Pantoea stewartii subsp. stewartii. Biosens Bioelectron 51:29–35
Chen L, Wang H, Guo T, Xiao C, Liu L, Zhang X, Liu B, Li P, Li B, Mao Y (2018) A rapid point-of-care test for dengue virus-1 based on a lateral flow assay with a near-infrared fluorescent dye. J Immunol Methods 456:23–27
Chang X, Zhang J, Wu L, Peng Y, Yang X, Li X, Ma A, Ma J, Chen G (2019) Research progress of near near-infrared fluorescence immunoassay. Micromachines 10:422–432
Sánchez-Purrà M, Carré-Camps M, Puig H, Bosch I, Gehrke L, Hamad-Schifferli K (2017) Surface-enhanced Raman spectroscopy-based sandwich immunoassays for multiplexed detection of zika and dengue viral biomarkers. ACS Infect Dis 3:767–776
Wang R, Kim K, Choi N, Wang X, Lee J, Jeon JH, Rhie G, Choo J (2018) Highly sensitive detection of high-risk bacterial pathogens using SERS-based lateral flow assay strips. Sensors Actuators B Chem 270:72–79
Liang T, Robinson R, Houghtaling J, Fridley G, Ramsey S (2016) Investigation of reagent delivery formats in a multivalent malaria sandwich immunoassay and implications for assay performance. Anal Chem 88:2311–2320
Qian S, Bau HH (2004) Analysis of lateral flow biodetectors: competitive format. Anal Biochem 326(2):211–224
Dufek EJ, Ehlert B, Granger MC, Sandrock TM, Legge SL, Herrmann MG, Meikle AW, Porter MD (2010) Competitive surface-enhanced Raman scattering assay for the 1,25-dihydroxy metabolite of vitamin D3. Analyst 135(11):2811–2817
Malone EM, Dowling G, Elliott CT, Kennedy DG, Regan L (2009) Development of a rapid, multi-class method for the confirmatory analysis of anti-inflammatory drugs in bovine milk using liquid chromatography tandem mass spectrometry. J Chromatogr A 1216:8132–8140
Guo Z, Park S, Yoon J, Shin I (2014) Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem Soc Rev 43:16–29
Technical manual of Millipore Corporation (2002) Rapid lateral flow test strips: considerations for product development:1–42
Tobita T, Oda M, Azuma T (2004) Segmental flexibility and avidity of IgM in the interaction of polyvalent antigen. Mol Immunol 40:803–811
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work is financially supported by Beijing Advanced Innovation Center for Food Nutrition and Human Health and the Chinese Universities Scientific Fund (2017304010328).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 601 kb).
Rights and permissions
About this article
Cite this article
Fan, R., Zhang, W., Jin, Y. et al. Lateral flow immunoassay for 5-hydroxyflunixin based on near-infrared fluorescence molecule as an alternative label to gold nanoparticles. Microchim Acta 187, 368 (2020). https://doi.org/10.1007/s00604-020-04338-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04338-z