Abstract
N-functionalization of pyrrole with carbon disulfide and subsequent chemical polymerization resulted in the development of a new sorbent material for the extraction of metals. The synthesized polymer, poly(pyrrole-N-carbodithioic acid) (PPy-CS2), is an air-stable, granular powder that is insoluble in water. PPy-CS2 combines pH-dependent chelation, extraction, and desorption sorbent properties that are exploited for the selective extraction and sensitive determination of heavy metals in water matrices using ultrasound-assisted dispersive micro solid-phase extraction and inductively coupled plasma mass spectrometry. Excellent removal and recovery of Cd(II), Co(II), Cu(II), Ni(II), Pb(II), and Zn(II) were achieved and compared with unfunctionalized polypyrrole, which demonstrated extraction resulted from chelation of the metal ions. The extraction efficiency of the PPy-CS2 sorbent as a function of pH, amount of sorbent, extraction time, and flow rate of the desorption solution were evaluated. Limits of detection ranged from 0.3 for cadmium to 11.2 ng/L for zinc with linear dynamic ranges from 0.1 to 500 μg/L and relative standard deviations from 2.2 to 6.3%. The sample preparation method was successfully applied for determination of the target metals in raw well water, treated well water, and river water. Validation was performed by analysis of a certified reference standard for trace metals in drinking water.

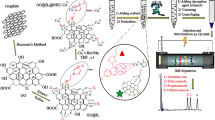
Schematic representation of the ultrasound-assisted micro solid-phase extraction protocol for the removal and recovery of heavy metals by the air-stable, granular, and reversible chelating polymer, poly (pyrrole-N-carbodithioic acid).





Similar content being viewed by others
References
Ma Y, Egodawatta P, McGree J, Liu A, Goonetilleke A (2016) Human health risk assessment of heavy metals in urban stormwater. Sci Total Environ 557-558:764–772. https://doi.org/10.1016/j.scitotenv.2016.03.067
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112(10):5073–5091. https://doi.org/10.1021/cr300133d
Shyam Sunder GS, Adhikari S, Rohanifar A, Poudel A, Kirchhoff JR (2020) Evolution of environmentally friendly strategies for metal extraction. Separations 7(1):4. https://doi.org/10.3390/separations7010004
Chauhan G, Pant KK, Nigam KD (2015) Chelation technology: a promising green approach for resource management and waste minimization. Environ Sci Process Impacts 17(1):12–40. https://doi.org/10.1039/C4EM00559G
Kanchi S, Singh P, Bisetty K (2014) Dithiocarbamates as hazardous remediation agent: a critical review on progress in environmental chemistry for inorganic species studies of 20th century. Arab J Chem 7(1):11–25. https://doi.org/10.1016/j.arabjc.2013.04.026
Hashemi B, Rezania S (2019) Carbon-based sorbents and their nanocomposites for the enrichment of heavy metal ions: a review. Microchim Acta 186(8):578. https://doi.org/10.1007/s00604-019-3668-2
Sitko R, Janik P, Zawisza B, Talik E, Margui E, Queralt I (2015) Green approach for ultratrace determination of divalent metal ions and arsenic species using total-reflection X-ray fluorescence spectrometry and mercapto-modified graphene oxide nanosheets as a novel adsorbent. Anal Chem 87(6):3535–3542. https://doi.org/10.1021/acs.analchem.5b00283
Liu Y, Qian P, Yu Y, Xiao L, Wang Y, Ye S, Chen Y (2017) Dithiocarbamate functionalized Al(OH)3-polyacrylamide adsorbent for rapid and efficient removal of Cu(II) and Pb(II). J Appl Polym Sci 134(46):45431. https://doi.org/10.1002/app.45431
Barfi B, Asghari A, Rajabi M (2020) Toward use of a nano layered double hydroxide/ammonium pyrrolidine dithiocarbamate in speciation analysis: one-step dispersive solid-phase extraction of chromium species in human biological samples. Arab J Chem 13(1):568–579. https://doi.org/10.1016/j.arabjc.2017.06.002
Tavares DS, Lopes CB, Daniel-da-Silva AL, Duarte AC, Trindade T, Pereira E (2014) The role of operational parameters on the uptake of mercury by dithiocarbamate functionalized particles. Chem Eng J 254:559–570. https://doi.org/10.1016/j.cej.2014.06.008
Bagheri H, Ayazi Z, Naderi M (2013) Conductive polymer-based microextraction methods: a review. Anal Chim Acta 767:1–13. https://doi.org/10.1016/j.aca.2012.12.013
Hasani T, Eisazadeh H (2013) Removal of Cd (II) by using polypyrrole and its nanocomposites. Synth Met 175:15–20. https://doi.org/10.1016/j.synthmet.2013.04.026
Li S, Lu X, Li X, Xue Y, Zhang C, Lei J, Wang C (2012) Preparation of bamboo-like PPy nanotubes and their application for removal of Cr(VI) ions in aqueous solution. J Colloid Interface Sci 378(1):30–35. https://doi.org/10.1016/j.jcis.2012.03.065
Chandra V, Kim KS (2011) Highly selective adsorption of Hg2+ by a polypyrrole–reduced graphene oxide composite. Chem Commun 47(13):3942–3944. https://doi.org/10.1039/C1CC00005E
Choi M, Jang J (2008) Heavy metal ion adsorption onto polypyrrole-impregnated porous carbon. J Colloid Interface Sci 325(1):287–289. https://doi.org/10.1016/j.jcis.2008.05.047
Aigbe UO, Das R, Ho WH, Srinivasu V, Maity A (2018) A novel method for removal of Cr(VI) using polypyrrole magnetic nanocomposite in the presence of unsteady magnetic fields. Sep Purif Technol 194:377–387. https://doi.org/10.1016/j.seppur.2017.11.057
Sahmetlioglu E, Yilmaz E, Aktas E, Soylak M (2014) Polypyrrole/multi-walled carbon nanotube composite for the solid phase extraction of lead(II) in water samples. Talanta 119:447–451. https://doi.org/10.1016/j.talanta.2013.11.044
Lim CW, Song K, Kim SH (2012) Synthesis of PPy/silica nanocomposites with cratered surfaces and their application in heavy metal extraction. J Ind Eng Chem 18(1):24–28. https://doi.org/10.1016/j.jiec.2011.11.115
Srivastava V, Maydannik P, Sharma YC, Sillanpää M (2015) Synthesis and application of polypyrrole coated tenorite nanoparticles (PPy@TN) for the removal of the anionic food dye ‘tartrazine’ and divalent metallic ions viz. Pb(II), Cd(II), Zn(II), Co(II), Mn(II) from synthetic wastewater. RSC Adv 5(98):80829–80843. https://doi.org/10.1039/C5RA14108G
Shin K-Y, Hong J-Y, Jang J (2011) Heavy metal ion adsorption behavior in nitrogen-doped magnetic carbon nanoparticles: isotherms and kinetic study. J Hazard Mater 190(1):36–44. https://doi.org/10.1016/j.jhazmat.2010.12.102
Young JA, Zhang C, Devasurendra AM, Tillekeratne LMV, Anderson JL, Kirchhoff JR (2016) Conductive polymeric ionic liquids for electroanalysis and solid-phase microextraction. Anal Chim Acta 910:45–52. https://doi.org/10.1016/j.aca.2016.01.017
Devasurendra AM, Zhang C, Young JA, Tillekeratne LMV, Anderson JL, Kirchhoff JR (2017) Electropolymerized pyrrole-based conductive polymeric ionic liquids and their application for solid-phase microextraction. ACS Appl Mater Interfaces 9(29):24955–24963. https://doi.org/10.1021/acsami.7b05793
Devasurendra AM, Palagama DSW, Rohanifar A, Isailovic D, Kirchhoff JR, Anderson JL (2018) Solid-phase extraction, quantification, and selective determination of microcystins in water with a gold-polypyrrole nanocomposite sorbent material. J Chromatogr A 1560:1–9. https://doi.org/10.1016/j.chroma.2018.04.027
Rohanifar A, Rodriguez LB, Devasurendra AM, Alipourasiabi N, Anderson JL, Kirchhoff JR (2018) Solid-phase microextraction of heavy metals in natural water with a polypyrrole/carbon nanotube/1, 10–phenanthroline composite sorbent material. Talanta 188:570–577. https://doi.org/10.1016/j.talanta.2018.05.100
Ramesh A, Rama Mohan K, Seshaiah K (2002) Preconcentration of trace metals on Amberlite XAD-4 resin coated with dithiocarbamates and determination by inductively coupled plasma-atomic emission spectrometry in saline matrices. Talanta 57(2):243–252. https://doi.org/10.1016/S0039-9140(02)00033-4
O'Riordan DMT, Wallace GG (1986) Poly(pyrrole-N-carbodithioate) electrode for electroanalysis. Anal Chem 58(1):128–131. https://doi.org/10.1021/ac00292a031
Andrew FP, Ajibade PA (2018) Metal complexes of alkyl-aryl dithiocarbamates: structural studies, anticancer potentials and applications as precursors for semiconductor nanocrystals. J Mol Struct 1155:843–855. https://doi.org/10.1016/j.molstruc.2017.10.106
Chougule M, Pawar S, Godse P, Mulik R, Sen S, Patil V (2011) Synthesis and characterization of polypyrrole (PPy) thin films. Soft Nanosci Lett 1(1):6–10. https://doi.org/10.4236/snl.2011.11002
Feist B, Sitko R (2019) Fast and sensitive determination of heavy metal ions as bathophenanthroline chelates in food and water samples after dispersive micro-solid phase extraction using graphene oxide as sorbent. Microchem J 147:30–36. https://doi.org/10.1016/j.microc.2019.03.013
Smirnova SV, Samarina TO, Ilin DV, Pletnev IV (2018) Multielement determination of trace heavy metals in water by microwave-induced plasma atomic emission spectrometry after extraction in unconventional single-salt aqueous biphasic system. Anal Chem 90(10):6323–6331. https://doi.org/10.1021/acs.analchem.8b01136
Yamini Y, Safari M (2018) Modified magnetic nanoparticles with catechol as a selective sorbent for magnetic solid phase extraction of ultra-trace amounts of heavy metals in water and fruit samples followed by flow injection ICP-OES. Microchem J 143:503–511. https://doi.org/10.1016/j.microc.2018.08.018
Yang B, Gong Q, Zhao L, Sun H, Ren N, Qin J, Xu J, Yang H (2011) Preconcentration and determination of lead and cadmium in water samples with a MnO2 coated carbon nanotubes by using ETAAS. Desalination 278(1):65–69. https://doi.org/10.1016/j.desal.2011.05.010
Su S, Chen B, He M, Hu B (2014) Graphene oxide–silica composite coating hollow fiber solid phase microextraction online coupled with inductively coupled plasma mass spectrometry for the determination of trace heavy metals in environmental water samples. Talanta 123:1–9. https://doi.org/10.1016/j.talanta.2014.01.061
Ghazaghi M, Shirkhanloo H, Mousavi HZ, Rashidi AM (2015) Ultrasound-assisted dispersive solid phase extraction of cadmium(II) and lead(II) using a hybrid nanoadsorbent composed of graphene and the zeolite clinoptilolite. Microchim Acta 182(7):1263–1272. https://doi.org/10.1007/s00604-015-1446-3
Sun J, Liang Q, Han Q, Zhang X, Ding M (2015) One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid phase extraction of heavy metal ions from biological samples. Talanta 132:557–563. https://doi.org/10.1016/j.talanta.2014.09.043
Sahraeian T, Sereshti H, Rohanifar A (2018) Simultaneous determination of bismuth, lead, and iron in water samples by optimization of USAEME and ICP-OES via experimental design. J Anal Test 2(1):98–105. https://doi.org/10.1007/s41664-017-0046-0
Acknowledgments
This manuscript is dedicated to our colleague and teacher, Professor Dean M. Giolando. Professor Donald Ronning is also acknowledged for providing raw and treated well water samples.
Funding
JRK would like to thank the National Science Foundation (NSF) for funding to purchase a scanning electron microscope (CHE–0840474) and renovation of Bowman-Oddy Laboratory to create the Center for Biosphere Restoration and Research (ARI–0963345). JRK also would like to acknowledge funding from the University of Toledo Research and Fellowship Program and the University of Toledo Center for Materials and Sensor Characterization for conducting ICP-MS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.36 mb)
Rights and permissions
About this article
Cite this article
Rohanifar, A., Alipourasiabi, N., Shyam Sunder, G.S. et al. Reversible chelating polymer for determination of heavy metals by dispersive micro solid-phase extraction with ICP-MS. Microchim Acta 187, 339 (2020). https://doi.org/10.1007/s00604-020-04308-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04308-5