Abstract
A magnetic polymer was molecularly imprinted with hippuric acid (HA) to obtain a nanomaterial with an architecture of type Fe3O4@SiO2@MIP. It was used as a sorbent for magnetic solid phase extraction of HA and methylhippuric acids (2-MHA, 3-MHA, 4-MHA) from urine samples. The respective imprinting factor are 4.6, 2.7, 2.0 and 1.8, respectively, and the selectivity coefficients are 1.7, 2.3 and 2.6. The effects of adsorbent amount, extraction time, pH value, ionic strength, desorption solvent and desorption time on the extraction of HA and MHA were investigated. Following elution with 1 mM NaOH solution, the 4 HAs were quantified by ultra-performance liquid chromatography with mass spectrometric detection. Under the optimal experimental conditions, the limits of detection (at S/N = 3) range between 89 ng·L−1 (for HA) and 170 ng·L−1 (for 4-MHA), the limits of quantification (at S/N = 10) range between 300 ng·L−1 (for HA) and 570 ng·L−1 (for 4-MHA), the linear range extends from 500 ng·L−1 to 10 mg·L−1, the relative standard deviations of intra-day range between 6.4 and 9.6% (for n = 6 at 10 μg·L−1) and inter-day range between 9.2 and 11.5% (for n = 6 at 10 μg·L−1). The method was successfully applied to the analysis of HA and MHA in (spiked) urine, and good recoveries were obtained.

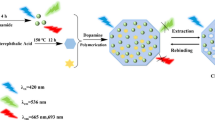
Schematic presentation of a method for the determination of hippuric acid (HA) and methylhippuric acid (MHA) in urine sample. The preparation of Fe3O4@SiO2@MIP includes three steps: (1) Preparation of Fe3O4 nanoparticles (NPs), (2) Preparation of Fe3O4@SiO2 and (3) Preparation of a molecular imprint in the surface of the nanoparticles (Fe3O4@SiO2@MIP). The MIP was used as a sorbent for magnetic solid phase extraction (MSPE) of the toluene exposure biomarkers.





Similar content being viewed by others
References
Yamazaki K, Tanaka E, Misawa S (1992) Urinary ortho-crsol concentrations as an indicator of toluene in halation in glue-sniffers. J Forensic Sci Soc 32:215–223. https://doi.org/10.1016/S0015-7368(92)73074-6
Anderson CE, Loomis GA (2003) Recognition and prevention of inhalant abuse. Am Fam Physician 68:869–874
Zinalibdin MR, Yacob AR (2013) Detection of hippuric acid: a glue solvent metabolite, using a mobile test kit. Arab J Chem 6:115–120. https://doi.org/10.1016/j.arabjc.2010.09.029
Arabi M, Ghaedi M, Ostovan A (2017) Water compatible molecularly imprinted nanoparticles as a restricted access material for extraction of hippuric acid, a biological indicator of toluene exposure, from human urine. Microchim Acta 184:879–887. https://doi.org/10.1007/s00604-016-2063-5
Penner N, Ramanathan R, Zgoda-Pols J, Chowdhury S (2010) Quantitative determination of hippuric and benzoic acids in urine by LC-MS/MS using surrogate standards. J Pharm Biomed Anal 52:534–543. https://doi.org/10.1016/j.jpba.2010.01.016
Ahmadi F, Asgharloo H, Sadeghi S, Gharehbagh-Aghababa V, Adibi H (2009) Post-derivatization procedure for determination of hippuric acid after extraction by an automated micro solid phase extraction system and monitoring by gas chromatography. J Chromatogr B 877:2945–2951. https://doi.org/10.1016/j.jchromb.2009.06.036
Ohashi Y, Mamiya T, Mitani K, Wang BL, Takigawa T, Kira S, Kataoka H (2006) Simultaneous determination of urinary hippuric acid, o-, m- and p-methylhippuric acids, mandelic acid and phenylglyoxylic acid for biomonitoring of volatile organic compounds by gas chromatography-mass spectrometry. Anal Chim Acta 566:167–171. https://doi.org/10.1016/j.aca.2006.03.018
Saito T, Takeichi S (2002) Simultaneous detection of hippuric acid and methylhippuric acid in urine by empore (TM) disk and gas chromatography-mass spectrometry. J Pharm Biomed Anal 30:365–370. https://doi.org/10.1016/S0731-7085(02)00268-6
Zuppi C, Rossetti DV, Vitali A, Vincenzoni F, Giardina B, Castagnola M, Messana I (2003) Determination of urinary hippuric acid by micellar electrokinetic capillary chromatography. J Chromatogr B 793:223–228. https://doi.org/10.1016/s1570-0232(03)00265-4
Zhao FY, Wang ZH, Wang H, Ding MY (2011) Determination of hippuric acid in human urine by ion chromatography with conductivity detection. J Chromatogr B 879:296–298. https://doi.org/10.1016/j.jchromb.2010.12.015
Kamiguchi H, Yamaguchi M, Murabayashi M, Mori I, Horinouchi A (2016) Method development and validation for simultaneous quantitation of endogenous hippuric acid and phenylacetylglycine in rat urine using liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J Chromatogr B 1035:76–83. https://doi.org/10.1016/j.jchromb.2016.09.036
Moein MM, El-Beqqali A, Javanbakht M, Karimi M, Akbari-adergani B, Abdel-Rehim M (2014) On-line detection of hippuric acid by microextraction with a molecularly-imprinted polysulfone membrane sorbent and liquid chromatography-tandem mass spectrometry. J Chromatogr A 1372:55–62. https://doi.org/10.1016/j.chroma.2014.10.061
Zhou QX, Wang YQ, Xiao JP, Fan HL (2016) Adsorption and removal of bisphenol a, alpha-naphthol and beta-naphthol from aqueous solution by Fe3O4@polyaniline core-shell nanomaterials. Synth Met 212:113–122. https://doi.org/10.1016/j.synthmet.2015.12.008
Yavuz E, Tokalioglu S, Patat S (2018) Core-shell Fe3O4 polydopamine nanoparticles as sorbent for magnetic dispersive solid-phase extraction of copper from food samples. Food Chem 263:232–239. https://doi.org/10.1016/j.foodchem.2018.04.134
Rahimi A, Zanjanchi MA, Bakhtiari S, Dehsaraei M (2018) Selective determination of caffeine in foods with 3D-graphene based ultrasound-assisted magnetic solid phase extraction. Food Chem 262:206–214. https://doi.org/10.1016/j.foodchem.2018.04.035
Fayazi M, Ghanei-Motlagh M (2017) Synthesis and application of novel modified magnetic nanocomposite for solid phase extraction of thallium(I) ions. Anal Bioanal Chem Res 4:189–200. https://doi.org/10.22036/abcr.2017.69558.1126
Ghanei-Motlagh M, Taher MA (2017) Magnetic silver(I) ion-imprinted polymeric nanoparticles on a carbon paste electrode for voltammetric determination of silver(I). Microchim Acta 184:1691–1699. https://doi.org/10.1007/s00604-017-2157-8
Amiri A, Baghayeri M, Sedighi M (2018) Magnetic solid-phase extraction of polycyclic aromatic hydrocarbons using a graphene oxide/Fe3O4@polystyrene nanocomposite. Microchim Acta 185:393. https://doi.org/10.1007/s00604-018-2928-x
Wang ZK, Zhao PF, Zhu BL, Jiang Z, Guo XJ (2018) Magnetic solid-phase extraction based on Fe3O4/graphene nanocomposites for enantioselective determination of representative profens in the environmental water samples and molecular docking study on adsorption mechanism of graphene. J Pharm Biomed Anal 156:88–96. https://doi.org/10.1016/j.jpba.2018.04.023
Tolmacheva VV, Apyari VV, Furletov AA, Dmitrienko SG, Zolotov YA (2016) Facile synthesis of magnetic hypercrosslinked polystyrene and its application in the magnetic solid-phase extraction of sulfonamides from water and milk samples before their HPLC determination. Talanta 152:203–210. https://doi.org/10.1016/j.talanta.2016.02.010
Musa M, Ibrahim WAW, Marsin FM, Keyon ASA, Nodeh HR (2018) Graphene-magnetite as adsorbent for magnetic solid phase extraction of 4-hydroxybenzoic acid and 3,4-dihydroxybenzoic acid in stingless bee honey. Food Chem 265:165–172. https://doi.org/10.1016/j.foodchem.2018.04.020
Ali LIA, Ibrahim WAW, Sulaiman AF, Kamboh MA, Sanagi MM (2016) New chrysin-functionalized silica-core shell magnetic nanoparticles for the magnetic solid phase extraction of copper ions from water samples. Talanta 148:191–199. https://doi.org/10.1016/j.talanta.2015.10.062
Nodeh HR, Ibrahim WAW, Kamboh MA, Sanagi MM (2017) New magnetic graphene-based inorganic-organic sol-gel hybrid nanocomposite for simultaneous analysis of polar and non-polar organophosphorus pesticides from water samples using solid-phase extraction. Chemosphere 166:21–30. https://doi.org/10.1016/j.chemosphere.2016.09.054
Liu HM, Li Z, Takafuji M, Ihara H, Qiu HD (2017) Octadecylimidazolium ionic liquid-modified magnetic materials: preparation, adsorption evaluation and their excellent application for honey and cinnamon. Food Chem 229:208–214. https://doi.org/10.1016/j.foodchem.2017.02.080
Xu XY, Liu RL, Guo PQ, Luo ZM, Cai X, Shu H, Ge YH, Chang C, Fu Q (2018) Fabrication of a novel magnetic mesoporous molecularly imprinted polymer based on pericarpium granati-derived carrier for selective absorption of bromelain. Food Chem 256:91–97. https://doi.org/10.1016/j.foodchem.2018.02.118
Hu MH, Huang PC, Suo LL, Wu FY (2018) Polydopamine-based molecularly imprinting polymers on magnetic nanoparticles for recognition and enrichment of ochratoxins prior to their determination by HPLC. Microchim Acta 185(6):300. https://doi.org/10.1007/s00604-018-2826-2
Ji WH, Sun RH, Geng YL, Liu W, Wang X (2018) Rapid, low temperature synthesis of molecularly imprinted covalent organic frameworks for the highly selective extraction of cyano pyrethroids from plant samples. Anal Chim Acta 1001:179–188. https://doi.org/10.1016/j.aca.2017.12.001
Ding H, Wang RY, Wang X, Ji WH (2018) Molecularly imprinted covalent organic polymers for the selective extraction of benzoxazole fluorescent whitening agents from food samples. J Sep Sci 41:3294–3301. https://doi.org/10.1002/jssc.201800540
Ji WH, Sun RH, Duan WJ, Wang X, Wang T, Mu Y, Guo LP (2017) Selective solid phase extraction of chloroacetamide herbicides from environmental water samples by amphiphilic magnetic molecularly imprinted polymers. Talanta 170:111–118. https://doi.org/10.1016/j.talanta.2017.04.005
Dramou P, Zuo PL, He H, Pham-Huy LA, Zou WY, Xiao DL, Pham-Huy C (2013) Development of novel amphiphilic magnetic molecularly imprinted polymers compatible with biological fluids for solid phase extraction and physicochemical behavior study. J Chromatogr A 1317:110–120. https://doi.org/10.1016/j.chroma.2013.07.075
Wang DD, Gao D, Xu WJ, Li F, Yin MN, Fu QF, Xia ZN (2018) Magnetic molecularly imprinted polymer for the selective extraction of hesperetin from the dried pericarp of Citrus reticulata Blanco. Talanta 184:307–315. https://doi.org/10.1016/j.talanta.2018.03.010
Zhu SS, Gan N, Pan DD, Li Y, Yang T, Hu FT, Cao YT, Wu DZ (2013) Extraction of tributyltin by magnetic molecularly imprinted polymers. Microchim Acta 180:545–553. https://doi.org/10.1007/s00604-013-0962-2
Tang YW, Gao JW, Liu XY, Lan JX, Gao X, Ma Y, Li M, Li JR (2016) Determination of ractopamine in pork using a magnetic molecularly imprinted polymer as adsorbent followed by HPLC. Food Chem 201:72–79. https://doi.org/10.1016/j.foodchem.2016.01.070
Ma ZY, Guan YP, Liu HZ (2005) Synthesis and characterization of micron-sized monodisperse superparamagnetic polymer particles with amino groups. J Polym Sci Polym Chem 43:3433–3439. https://doi.org/10.1002/pola.20803
Acknowledgements
This project was supported by the National Nature Science Foundation for Young Scholars of China (No. 21505007), and Changsha science and technology project (No. kq1701023).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 228 kb)
Rights and permissions
About this article
Cite this article
Hu, C., Yang, Z., Yan, F. et al. Extraction of the toluene exposure biomarkers hippuric acid and methylhippuric acid using a magnetic molecularly imprinted polymer, and their quantitation by LC-MS/MS. Microchim Acta 186, 135 (2019). https://doi.org/10.1007/s00604-019-3239-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3239-6