Abstract
Background
The association between thiopurine use and testicular reproductive functions remains unclear. In this study, we investigated whether thiopurines affect testicular functions based on the NUDT15 genotypes using Nudt15R138C knock-in mice.
Methods
The male Nudt15R138C knock-in mice (9–12 weeks) were treated with mercaptopurine (MP: 0.5 mg/kg/day) for 4 or 12 weeks. To examine reversibility, some mice were maintained for a further 12 weeks under MP-free condition.
Results
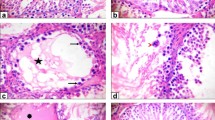
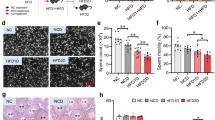
After MP treatment for 4 weeks, Nudt15R138C/R138C mice exhibited a significant reduction of testis weight compared to Nudt15+/+ mice and Nudt15+/R138C mice. The epithelial height and diameter of seminiferous tubules were significantly reduced in Nudt15R138C/R138C mice compared to Nudt15+/+ and Nudt15+/R138C mice. Apoptotic cells were significantly increased in Nudt15R138C/R138C mice, and most of apoptotic cells were spermatogonia. There were no significant changes in sperm counts and sperm morphology in MP-treated Nudt15R138C/R138C mice after 4-week MP treatment. On the other hand, after MP treatment for 12 weeks, the Nudt15+/R138C mice, but not Nudt15+/+ mice, exhibited a significant reduction in the testis weight and atrophic changes of seminiferous tubules, but these changes disappeared after 12-week rearing under MP-free condition. Despite a significant increase in abnormal sperm rate, there were no changes in the ability to conceive. No differences in serum levels of follicle-stimulating hormone or testosterone were observed between MP-treated Nudt15+/R138C and Nudt15+/+ mice after 12-week MP treatment.
Conclusions
Thiopurines exert harmful effects on testicular reproductive function according to host NUDT15 genotypes.







Similar content being viewed by others
References
Amin J, Huang B, Yoon J, et al. Update 2014: advances to optimize 6-mercaptopurine and azathioprine to reduce toxicity and improve efficacy in the management of IBD. Inflamm Bowel Dis. 2015;21:445–52.
Lee MN, Kang B, Choi SY, et al. Relationship between azathioprine dosage, 6-thioguanine nucleotide levels, and therapeutic response in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis. 2015;21:1054–62.
Timmer A, McDonald JW, Tsoulis DJ, et al. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;9:CD000478.
Chande N, Patton PH, Tsoulis DJ, et al. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2015;10:CD000067.
Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14:4–22.
van Gennep S, de Boer NK, D’Haens GR, et al. Thiopurine treatment in ulcerative colitis: a critical review of the evidence for current clinical practice. inflamm Bowel Dis. 2017;24:67–77.
Gearry RB, Barclay ML, Burt MJ, et al. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2004;13:563–7.
Van Dieren JM, Hansen BE, Kuipers EJ, et al. Meta-analysis: inosine triphosphate pyrophosphatase polymorphisms and thiopurine toxicity in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2007;26:643–52.
Chaparro M, Ordas I, Cabre E, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404–10.
Chang JY, Cheon JH. Thiopurine therapy in patients with inflammatory bowel disease: a focus on metabolism and pharmacogenetics. Dig Dis Sci. 2019;64:2395–403.
Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017–20.
Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367–73.
Relling MV, Schwab M, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. 2019;105:1095–105.
Kakuta Y, Kawai Y, Okamoto D, et al. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: a multicenter study. J Gastroenterol. 2018;53:1065–78.
Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33:1235–42.
Chang JY, Park SJ, Jung ES, et al. Genotype-based treatment with thiopurine reduces incidence of myelosuppression in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18(2010–8): e2.
Asada A, Nishida A, Shioya M, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016;51:22–9.
Kakuta Y, Naito T, Onodera M, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016;16:280–5.
Warner B, Johnston E, Arenas-Hernandez M, et al. A practical guide to thiopurine prescribing and monitoring in IBD. Frontline Gastroenterol. 2018;9:10–5.
Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56:489–526.
Imai T, Kawahara M, Tatsumi G, et al. Thiopurine use during pregnancy has deleterious effects on offspring in Nudt 15(R138C) knock-in mice. Cell Mol Gastroenterol Hepatol. 2021;12:335–7.
Tatsumi G, Kawahara M, Imai T, et al. Thiopurine-mediated impairment of hematopoietic stem and leukemia cells in Nudt 15(R138C) knock-in mice. Leukemia. 2020;34:882–94.
Yang L, Boyd K, Kaste SC, et al. A mouse model for glucocorticoid-induced osteonecrosis: effect of a steroid holiday. J Orthop Res. 2009;27:169–75.
Shin SC, Kang YM, Jin YW, et al. Relative morphological abnormalities of sperm in the caudal epididymis of high- and low-dose-rate gamma-irradiated ICR mice. J Radiat Res. 2009;50:261–6.
Takeda N, Yoshinaga K, Furushima K, et al. Viable offspring obtained from Prm1-deficient sperm in mice. Sci Rep. 2016;6:27409. https://doi.org/10.1038/srep27409.
Yoshida S. From cyst to tubule: innovations in vertebrate spermatogenesis. Wiley Interdiscip Rev Dev Biol. 2016;5:119–31.
Weinbauer GF, Nieschlag E. Gonadotrophin control of testicular germ cell development. Adv Exp Med Biol. 1995;377:55–65.
Migrenne S, Moreau E, Pakarinen P, et al. Mouse testis development and function are differently regulated by follicle-stimulating hormone receptors signaling during fetal and prepubertal life. PLoS ONE. 2012;7: e53257. https://doi.org/10.1371/journal.pone.0053257.
Patel H, Bhartiya D. Direct action of FSH on testicular stem cells. Stem Cell Res Ther. 2019;10:261.
Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–7.
van den Heuvel TR, Wintjens DS, Jeuring SF, et al. Inflammatory bowel disease, cancer and medication: cancer risk in the Dutch population-based IBDSL cohort. Int J Cancer. 2016;139:1270–80.
Oatley JM, Reeves JJ, McLean DJ. Biological activity of cryopreserved bovine spermatogonial stem cells during in vitro culture. Biol Reprod. 2004;71:942–7.
Habas K, Brinkworth MH, Anderson D. In vitro responses to known in vivo genotoxic agents in mouse germ cells. Environ Mol Mutagen. 2017;58:99–107.
Kotaja N, De Cesare D, Macho B, et al. Abnormal sperm in mice with targeted deletion of the act (activator of cAMP-responsive element modulator in testis) gene. Proc Natl Acad Sci U S A. 2004;101:10620–5.
Simsek M, Lambalk CB, Wilschut JA, et al. The associations of thiopurines with male fertility and paternally exposed offspring: a systematic review and meta-analysis. Hum Reprod Update. 2018;24:192–206.
Ligumsky M, Badaan S, Lewis H, et al. Effects of 6-mercaptopurine treatment on sperm production and reproductive performance: a study in male mice. Scand J Gastroenterol. 2005;40:444–9.
Karl PI, Katz R, Daum F, et al. 6-Mercaptopurine and spermatogenesis in the young rat. Dig Dis Sci. 1991;36:1569–73.
de Boer NK, de Meij T, van Bodegraven AA. Thiopurines during pregnancy in inflammatory bowel disease: is there a risk for the (unborn) child? Expert Rev Gastroenterol Hepatol. 2013;7:669–71.
Coelho J, Beaugerie L, Colombel JF, et al. Pregnancy outcome in patients with inflammatory bowel disease treated with thiopurines: cohort from the CESAME Study. Gut. 2011;60:198–203.
Andoh A, Kawahara M, Imai T, et al. Thiopurine pharmacogenomics and pregnancy in inflammatory bowel disease. J Gastroenterol. 2021;56:881–90.
Akbari M, Shah S, Velayos FS, et al. Systematic review and meta-analysis on the effects of thiopurines on birth outcomes from female and male patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:15–22.
Gubatan J, Barber GE, Nielsen OH, et al. Paternal medications in inflammatory bowel disease and male fertility and reproductive outcomes: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023;21:2222–38.
Friedman S, Larsen MD, Magnussen B, et al. Paternal use of azathioprine/6-mercaptopurine or methotrexate within 3 months before conception and long-term health outcomes in the offspring-A nationwide cohort study. Reprod Toxicol. 2017;73:196–200.
Acknowledgements
The authors wish to thank DR. Keiji Tomita, Prof. Susumu Kageyama and Prof. Akihiro Kawauchi (Department of Urology, Shiga University of Medical Science) for professional opinions and helpful discussion.
Funding
This work was supported by the Japan Agency for Medical Research and Development (AMED) under grant number JP23ek0410091 (YK), and in part by a Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan under grant number 22K08054 (AA), and in part by Grants from the Japan Sciences Research Grant for Research on Intractable Diseases (Japanese Inflammatory Bowel Disease Research Group) affiliated with the Japan Ministry of Health, Labour and Welfare under grant number 20FC1027.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, performing experiments, data collection and analysis were performed by YY and TI. The first draft of the manuscript was written by YY and AA, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AA receiving lecture fee from Takeda Pharmaceutical Co. Ltd., AbbVie GK, and Miyarisan Pharmaceutical Co. Ltd. YK received patent royalties from Medical & Biological Laboratories Co., Ltd, and lecture fee from Takeda Pharmaceutical Co. Ltd., AbbVie GK, and Janssen Pharmaceutical K.K. All other authors declare that they have no conflict of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yokota, Y., Imai, T., Kawahara, M. et al. Thiopurines exert harmful effects on spermatogenesis in Nudt15R138C knock-in mice. J Gastroenterol 59, 109–118 (2024). https://doi.org/10.1007/s00535-023-02059-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-023-02059-7