Abstract
Background and Aims
The usefulness of APRI or FIB-4 is well established as a non-invasive liver fibrosis marker at a point of diagnosis in patients with chronic liver disease. However, their applicability for the monitoring of progression of liver fibrosis over time is yet to be determined. We aimed to clarify the feasibility of APRI and FIB-4 for the longitudinal evaluation of liver fibrosis in patients with chronic hepatitis B and C.
Methods
This is a multi-center retrospective and prospective cohort study, enrolling 1029 patients with HCV and 384 patients with HBV who were histologically diagnosed by liver biopsy. The observation period of retrospective and prospective study was 14 and 12 years, respectively. The APRI and FIB-4 were traced back in cases of histologically diagnosed cirrhosis, and those were prospectively analyzed after biopsy in cases diagnosed as F3 of METAVIR score, respectively.
Results
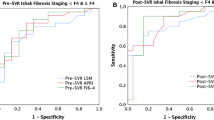
The averaged APRI and FIB-4 exhibited time-dependent increase in the retrospective study of hepatitis C patients (increase by 0.09/year in APRI and 0.29/year in FIB-4). In the prospective study of untreated hepatitis C patients, such increases were 0.14/year in APRI and 0.40/year in FIB-4, respectively. Neither the average of APRI nor FIB-4 showed a specific tendency with hepatitis B patients and treatment-experienced hepatitis C patients.
Conclusion
The APRI and FIB-4 may serve as a transition indicator of liver fibrosis in anti-viral treatment-naïve patients with chronic hepatitis C.





Similar content being viewed by others
Abbreviations
- APRI:
-
AST to platelet ratio index
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- ROC analysis:
-
Receiver operating characteristic analysis
References
Seto WK, Lo YR, Pawlotsky JM, et al. Chronic hepatitis B virus infection. Lancet. 2018;392:2313–24.
Trivedi HD, Patwardhan VR, Malik R. Chronic hepatitis C infection—Noninvasive assessment of liver fibrosis in the era of direct acting antivirals. Dig Liver Dis. 2019;51:183–9.
Singh S, Muir AJ, Dieterich DT, et al. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152:1544–77.
World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. https://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/. Accessed 31 July 2020.
World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. https://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed 31 July 2020.
Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–99.
AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance. Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2019;2020(71):686–721.
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98.
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511.
Japanese Guideline for Management of Hepatitis B ver 3.1 (written in Japanese). The Japan Society of Hepatology. 2019. https://www.jsh.or.jp/medical/guidelines/jsh_guidlines/hepatitis_b. Accessed 31 July 2020.
Japanese Guideline for Management of Hepatitis C ver 7 (written in Japanese). The Japan Society of Hepatology. 2019. http://www.jsh.or.jp/medical/guidelines/jsh_guidlines/hepatitis_c. Accessed 31 July 2020.
Papastergiou V, Tsochatzis E, Burroughs AK. Non-invasive assessment of liver fibrosis. Ann Gastroenterol. 2012;25:218–31.
Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-68.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Lu M, Li J, Zhang T, Chronic hepatitis cohort study investigators, et al. Serum biomarkers indicate long-term reduction in liver fibrosis in patients with sustained virological response to treatment for HCV infection. Clin Gastroenterol Hepatol. 2016;14:1044-1055.e3.
Schmid P, Bregenzer A, Huber M, Swiss HIV cohort study, et al. Progression of liver fibrosis in HIV/HCV co-infection: a comparison between non-invasive assessment methods and liver biopsy. PLoS ONE. 2015;10:e0138838.
Hiramatsu N, Hayashi N, Kasahara A, et al. Improvement of liver fibrosis in chronic hepatitis C patients treated with natural interferon alpha. J Hepatol. 1995;22:135–42.
Elsharkawy A, Alem SA, Fouad R, et al. Changes in liver stiffness measurements and fibrosis scores following sofosbuvir based treatment regimens without interferon. J Gastroenterol Hepatol. 2017;32:1624–30.
Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292–302.
Xu XY, Kong H, Song RX, et al. The effectiveness of noninvasive biomarkers to predict hepatitis B-related significant fibrosis and cirrhosis: a systematic review and meta-analysis of diagnostic test accuracy. PLoS ONE. 2014;9:e100182.
Xu XY, Wang WS, Zhang QM, et al. Performance of common imaging techniques vs serum biomarkers in assessing fibrosis in patients with chronic hepatitis B: A systematic review and meta-analysis. World J Clin Cases. 2019;7:2022–37.
Zhang Z, Wang G, Kang K, et al. The diagnostic accuracy and clinical utility of three noninvasive models for predicting liver fibrosis in patients with HBV infection. PLoS ONE. 2016;11:e0152757.
Li Q, Ren X, Lu C, et al. Evaluation of APRI and FIB-4 for noninvasive assessment of significant fibrosis and cirrhosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN: A retrospective cohort study. Medicine (Baltimore). 2017;96:e6336.
Sanai FM, Farah T, Albeladi K, et al. Diminished accuracy of biomarkers of fibrosis in low replicative chronic hepatitis B. BMC Gastroenterol. 2017;17:101.
Kim WR, Berg T, Asselah T, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64:773–80.
Stasi C, Salomoni E, Arena U, et al. Non-invasive assessment of liver fibrosis in patients with HBV-related chronic liver disease undergoing antiviral treatment: a preliminary study. Eur J Pharmacol. 2017;806:105–9.
Li J, Gordon SC, Rupp LB, et al. CHeCS Investigators. Long-term progression of viral load and serum markers of fibrosis among treated and untreated patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32:1250–7.
Graf C, Mondorf A, Knop V, et al. Evaluation of point shear wave elastography using acoustic radiation force impulse imaging for longitudinal fibrosis assessment in patients with HBeAg-negative HBV infection. J Clin Med. 2019;8:2101.
Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2:100067.
Hagström H, Talbäck M, Andreasson A, et al. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol. 2020;73:1023–9.
Acknowledgements
We are grateful to the doctors in the following institutes for their support during this study: the Hospital of Hyogo College of Medicine, Hiroshima University Hospital, Hokkaido University Hospital, Yamagata University Hospital, University of Yamanashi Hospital, Osaka City University Hospital, Kumamoto University Hospital, and Kurume University Hospital.
Funding
This work was supported by the Japan Agency for Medical Research and Development. This study was conducted as a part of the Policy Research for Hepatitis Measures of the Ministry of Health, Labor and Welfare in Japan and was supported by Health, Labour and Welfare Sciences Research Grants in Japan (Grant number H29- kansei-shitei-001 and 20HC2002).
Author information
Authors and Affiliations
Contributions
Conceptualization: JI, MK and TK. Methodology: JI, MK and TK. Formal analysis: JI. Investigation: JI, HS, NO and MK. Resources: JI, HS, TS, NO, MK, MT, TT, NS, NE, YU, NK, SK, SN, KC, and JT. Data curation: JI, HS. Writing (original draft preparation): JI. Writing (review and editing): MK and TK. Supervision: MK and TK. Project administration: TK. Funding acquisition: TK.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Itakura, J., Kurosaki, M., Setoyama, H. et al. Applicability of APRI and FIB-4 as a transition indicator of liver fibrosis in patients with chronic viral hepatitis. J Gastroenterol 56, 470–478 (2021). https://doi.org/10.1007/s00535-021-01782-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-021-01782-3