Abstract
Purpose
Colorectal cancer (CRC) incidence and mortality are increasing among young adults (YAs) aged 18–39. This study compared quality of life (QOL) between YA and older adult CRC survivors in the ColoCare Study.
Methods
Participants were grouped by age (years) as follows: 18–39 (YA), 40–49, 50–64, and 65 + . Functional QOL (physical, social, role, emotional, cognitive) and global QOL were assessed with the EORTC-QLQ-C30 at enrollment, 3, 6, and 12 months. Average scores were compared between groups over time using longitudinal mixed-effect modeling. Proportions with clinically meaningful QOL impairment were calculated using age-relevant thresholds and compared between groups over time using logistic regression with mixed effects.
Results
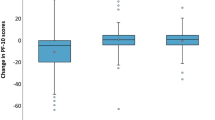
Participants (N = 1590) were n = 81 YAs, n = 196 aged 40–49, n = 627 aged 50–64, and n = 686 aged 65 + . Average physical function was better among YAs than participants aged 50–64 (p = 0.010) and 65 + (p < 0.001), and average social function was worse among YAs than aged 65 + (p = 0.046). Relative to YAs, all age groups were less likely to report clinically meaningful social dysfunction (aged 40–49 OR = 0.13, 95%CI = 0.06–0.29; aged 50–64 OR = 0.10, 95%CI = 0.05–0.21; aged 65 + OR = 0.07, 95%CI = 0.04–0.15) and role dysfunction (aged 40–49 OR = 0.36, 95%CI = 0.18–0.75; aged 50–64 OR = 0.41, 95%CI = 0.22–0.78; aged 65 + OR = 0.32, 95%CI = 0.17–0.61). Participants aged 40–49 were also less likely to report physical dysfunction (OR = 0.42, 95%CI = 0.19–0.93).
Conclusion
YA CRC survivors reported better physical and worse social function compared to older CRC survivors, and YA CRC survivors were more likely to report clinically meaningful social, role, and physical disfunction. Future work should further investigate QOL using age-relevant benchmarks to inform best practices for CRC survivorship care.
Trial registration
NCT02328677, registered December 2014.

Similar content being viewed by others
Data availability
The ColoCare Study data and code are available from the corresponding author upon reasonable request and as described on the ColoCare website: (https://uofuhealth.utah.edu/huntsman/labs/colocare-consortium/). Our data sharing procedures have been updated and are available online: (https://uofuhealth.utah.edu/huntsman/labs/colocare-consortium/data-sharing/new-projects.php). For additional questions, please contact the ColoCare Study Administrator Team (colocarestudy_admin@hci.utah.edu).
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- EORTC-QLQ-C30:
-
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire
- OR:
-
Odds ratio
- QOL:
-
Quality of life
- SE:
-
Standard error
- YA:
-
Young adult
References
American Cancer Society (2023) Cancer facts & figures 2023. American Cancer Society, Atlanta
American Cancer Society (2023) Colorectal cancer facts & figures 2023–2025. American Cancer Society, Atlanta, GA
American Cancer Society (2020) Cancer facts and figures 2020 special section: cancer in adolescent and young adults. American Cancer Society, Atlanta, GA
Spaander MC, Zauber AG, Syngal S, Blaser MJ, Sung JJ, You YN et al (2023) Young-onset colorectal cancer. Nat Rev Dis Primers 9(1):21
Patterson P, McDonald FEJ, Zebrack B, Medlow S (2015) Emerging issues among adolescent and young adult cancer survivors. Semin Oncol Nurs 31(1):53–59
Zebrack BJ (2011) Psychological, social, and behavioral issues for young adults with cancer. Cancer 117(10 Suppl):2289–2294
Quinn GP, Gonçalves V, Sehovic I, Bowman ML, Reed DR (2015) Quality of life in adolescent and young adult cancer patients: a systematic review of the literature. Patient Relat Outcome Meas 6:19
Sodergren SC, Husson O, Robinson J, Rohde GE, Tomaszewska IM, Vivat B et al (2017) Systematic review of the health-related quality of life issues facing adolescents and young adults with cancer. Qual Life Res 26:1659–1672
Lang MJ, Giese-Davis J, Patton SB, Campbell DJ (2018) Does age matter? Comparing post-treatment psychosocial outcomes in young adult and older adult cancer survivors with their cancer-free peers. Psychooncology 27(5):1404–1411
Perl G, Nordheimer S, Lando S, Benedict C, Brenner B, Perry S et al (2016) Young patients and gastrointestinal (GI) tract malignancies-are we addressing the unmet needs? BMC Cancer 16:1–8
Miller KA, Stal J, Gallagher P, Weng Z, Freyer DR, Kaslander JN et al (2021) Time from diagnosis and correlates of health-related quality of life among young adult colorectal cancer survivors. Cancers 13(16):4045
Khoo AMG, Lau J, Loh XS, Ng CWT, Griva K, Tan KK (2022) Understanding the psychosocial impact of colorectal cancer on young-onset patients: A scoping review. Cancer Med 11(7):1688–1700
Waddell O, Mclauchlan J, McCombie A, Glyn T, Frizelle F (2023) Quality of life in early-onset colorectal cancer patients: systematic review. BJS open. 7:zrad030
Ulrich CM, Gigic B, Böhm J, Ose J, Viskochil R, Schneider M et al (2019) The ColoCare Study: a paradigm of transdisciplinary science in colorectal cancer outcomes. Cancer Epidemiol Biomarkers Prev 28(3):591–601
Gigic B, Boeing H, Toth R, Böhm J, Habermann N, Scherer D et al (2018) Associations between dietary patterns and longitudinal quality of life changes in colorectal cancer patients: the ColoCare study. Nutr Cancer 70(1):51–60
Gigic B, Nattenmüller J, Schneider M, Kulu Y, Syrjala KL, Böhm J et al (2020) The role of CT-quantified body composition on longitudinal health-related quality of life in colorectal cancer patients: the Colocare study. Nutrients 12(5):1247
Vardy J, Dhillon H, Pond G, Renton C, Dodd A, Zhang H et al (2016) Fatigue in people with localized colorectal cancer who do and do not receive chemotherapy: a longitudinal prospective study. Ann Oncol 27(9):1761–1767
Li X, Hoogland AI, Small BJ, Crowder SL, Gonzalez BD, Oswald LB et al (2023) Trajectories and risk factors of fatigue following colorectal cancer diagnosis. Colorectal Dis 25(10):2054–2063
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–76
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A et al (2001) The EORTC QLQ-C30 scoring manual, 3rd edn. European Organisation for Research and Treatment of Cancer, Brussels, p 2001
Lidington E, Giesinger JM, Janssen SHM, Tang S, Beardsworth S, Darlington AS et al (2022) Identifying health-related quality of life cut-off scores that indicate the need for supportive care in young adults with cancer. Qual Life Res 31(9):2717–2727
Giesinger JM, Loth FLC, Aaronson NK, Arraras JI, Caocci G, Efficace F et al (2020) Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J Clin Epidemiol 118:1–8
Nolte S, Liegl G, Petersen MA, Aaronson NK, Costantini A, Fayers PM et al (2019) General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer 107:153–163
Kwok OM, Underhill AT, Berry JW, Luo W, Elliott TR, Yoon M (2008) Analyzing longitudinal data with multilevel models: an example with individuals living with lower extremity intra-articular fractures. Rehabil Psychol 53(3):370–386
Cohen J. Statistical power analysis for the behavioral sciences. 2 ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988
Chen H, Cohen P, Chen S (2010) How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat B: Simul Comput 39(4):860–4
Pearman T, Yanez B, Peipert J, Wortman K, Beaumont J, Cella D (2014) Ambulatory cancer and US general population reference values and cutoff scores for the functional assessment of cancer therapy. Cancer 120(18):2902–2909
Cavallo J (2020) The ASCO post [Internet] 2020. Trends to watch in early-onset cancer among young adults. [cited 2023 Nov]. Available from: https://ascopost.com/issues/october-10-2020/trends-to-watch-in-early-onset-cancer-among-young-adults/#:~:text=for%20younger%20patients.%E2%80%9D-,Dr.,treatment%2C%20allowing%20for%20improved%20outcomes
Berghuijs KMvT, Kaddas HK, Trujillo G, Rouhani G, Chevrier A, Ose J et al (2023) Age-related differences in employment, insurance, and financial hardship among colorectal cancer patients: a report from the ColoCare Study. J Cancer Surviv. ePub 2023 March. Available from https://doi.org/10.1007/s11764-023-01362-9
Andersen NH, Christiansen JA, la Cour K, Aagesen M, Tang LH, Joergensen DS et al (2022) Differences in functioning between young adults with cancer and older age groups: a cross-sectional study. Eur J Cancer Care 31(6):e13660
Patel VR, Hussaini SQ, Blaes AH, Morgans AK, Haynes AB, Adamson AS et al (2023) Trends in the prevalence of functional limitations among US cancer survivors, 1999–2018. JAMA Oncol 9(7):1001–1003
Himbert C, Figueiredo JC, Shibata D, Ose J, Lin T, Huang LC et al (2021) Clinical characteristics and outcomes of colorectal cancer in the ColoCare study: differences by age of onset. Cancers 13(15):3817
Acknowledgements
The authors would like to gratefully acknowledge Sofia Cobos, MPH, Julaxis Love, and Devon Conant for their assistance with data collection. The authors would also like to gratefully acknowledge the colorectal cancer survivors who participated in this study.
Funding
This study was supported in part by an Innovation Award via the Moffitt Cancer Center Department of Epidemiology (MPIs: Siegel, Oswald), institutional funds via the Moffitt Cancer Center AYA Program, Florida Department of Health Bankhead Coley Award (09BN-13, Siegel), and funding via the National Cancer Institute of the National Institutes of Health (U01CA206110, R01NR018762, R01CA189184, R01CA207371, R01CA254108). The ColoCare Study at the Heidelberg site was supported by the German Ministry of Education and Research projects 01KT1503 and 01KD2101D, the National Institutes of Health/National Cancer Institute (NHI/NCI) projects R01 CA189184 and U01 CA206110, the Stiftung LebensBlicke, the Matthias-Lackas Foundations, the Rahel-Goitein-Straus-Program, Medical Faculty Heidelberg University, and the Heidelberger Stiftung Chirurgie, Heidelberg University Hospital. This work was also supported in part by the Total Cancer Care Protocol and the Participant Research, Interventions, and Measurements Core at Moffitt Cancer Center, an NCI designated Comprehensive Cancer Center (P30CA076292).
Author information
Authors and Affiliations
Contributions
LBO: conceptualization, funding acquisition, writing – original draft, writing – review and editing; AB: data curation, formal analysis, writing – original draft, writing – review and editing; XL: data curation, formal analysis, visualization, writing – original draft, writing – review and editing; EJ-B: project administration, writing – review and editing; GT: project administration, investigation, writing – review and editing; SF: project administration, writing – review and editing; BJS: data curation, writing – review and editing; JO: project administration, investigation, writing – review and editing; SH: project administration, writing – review and editing; IS: project administration, writing – review and editing; LCH: resources, writing – review and editing; KM: writing – review and editing; MGM: resources, writing – review and editing; DC: project administration, writing – review and editing; SAC: resources, writing – review and editing; MK: resources, writing – review and editing; EHW: resources, writing – review and editing; VD: project administration, writing – review and editing; NCL: project administration, writing – review and editing; JG: resources, writing – review and editing; ATT: conceptualization, funding acquisition, writing – review and editing; CIL: conceptualization, funding acquisition, writing – review and editing; DS: conceptualization, funding acquisition, writing – review and editing; MS: conceptualization, funding acquisition, writing – review and editing; BG: conceptualization, funding acquisition, writing – review and editing; JCF: conceptualization, funding acquisition, writing – review and editing; HSLJ: conceptualization, funding acquisition, writing – review and editing; CMU: conceptualization, funding acquisition, writing – review and editing; EMS: conceptualization, funding acquisition, writing – original draft, writing – review and editing.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the Declaration of Helsinki. Approval was granted by the ethics committee of each recruitment site: Moffitt Cancer Center (USF104189, USF108437), University of Tennessee Health Science Center (16–04626-FB), Washington University School of Medicine (201610032), Huntsman Cancer Institute (IRB_00077147), Cedars-Sinai Medical Center (Pro000464423, CR00012892), Fred Hutchinson Cancer Center (6407), and University of Heidelberg (300/2001, S-134/2016).
Consent to participate and publication
Informed consent to participate and to have de-identified and aggregate study data published was obtained from all individual participants included in this study.
Competing interests
Dr. Felder reports an advisory role for GSK and research funding from ViewRay and Natera. Dr. Cohen reports personal fees and grants from Pfizer and personal fees from Taiho, Bayer, Regeneron, Biomea, Eisai, Delcath, Isofol, and GSK. Dr. Gong reports advisory/consulting for Aveo, Basilea, Bayer, EMD Serono, Elsevier, Exelixis, HalioDx, Janssen, Pfizer, Inc, QED Therapeutics, Seagen, and Taiho. Dr. Jim reports grant funding from Kite Pharm and a consultant role for SBR Bioscience. Dr. Ulrich reports oversight over research funded by several pharmaceutical companies in her role as Cancer Center Director, but she has not received funding directly herself. The other authors have no competing interests to disclose.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Laura B. Oswald and Amanda Bloomer contributed equally.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oswald, L.B., Bloomer, A., Li, X. et al. Functional quality of life among newly diagnosed young adult colorectal cancer survivors compared to older adults: results from the ColoCare Study. Support Care Cancer 32, 298 (2024). https://doi.org/10.1007/s00520-024-08511-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08511-5